Annotation H: The vulvar epithelium
Click here for Key Points to AnnotationThe patient has presented with a problem, usually a complaint of vulvovaginal itching, burning, other types of pain, lumps or bumps, abnormal discharge, abnormal odor, dyspareunia, or other sexual difficulty. You have examined the vulvar tissue for anatomical abnormalities. (Annotation F: The vulvar architecture). Now you must examine the skin itself.
Why there is difficulty in diagnosing women with vulvovaginal complaints
One of the reasons that women with vulvovaginal complaints are not correctly diagnosed and treated is that the two fields of gynecology and dermatology collide in the area of the female reproductive tract, but they do not “speak” to each other. Dermatologists who do “complete” skin checks often have women leave their underwear on, expecting the gynecologist to inspect the vulvar skin during routine exams. If a woman complains to a dermatologist about itching or burning, the gynecological (vaginal) part of the exam may be omitted. Meanwhile, gynecologists have been taught to protect the woman’s modesty by not inspecting the external tissue too extensively and they have not been taught very much dermatology. If a woman complains to a gynecologist about itching or burning, often yeast or bacterial vaginosis is suspected and the dermatological (vulvar skin) part of the exam may be omitted.It is assumed that changes in normal architecture are due to normal variants or, in the case of the postmenopausal woman, menopause.
Vulvovaginal specialists have become experienced in both fields (gynecology and dermatology), at least in the arena of the female genitalia. We believe that this more complete approach should not be restricted to specialists, but should be a routine part of medical and advanced practice nursing or physician assistant education for clinicians providing women’s health care.
Because the vulvovaginal epithelium is contiguous with the rest of the woman’s skin, some understanding of the basic anatomy and physiology of the skin prior to discussion of the vulvar epithelium itself, will greatly help the women’s health practitioner to diagnose in the vulvovaginal area. Descriptions of normal epithelial cell life will also help you understand the wet prep done to evaluate the vagina. (Annotation P: Vaginal secretions, pH, microscopy and cultures). As you grow in your care of women with vulvovaginal problems, you will better comprehend how important these fundamental concepts are.
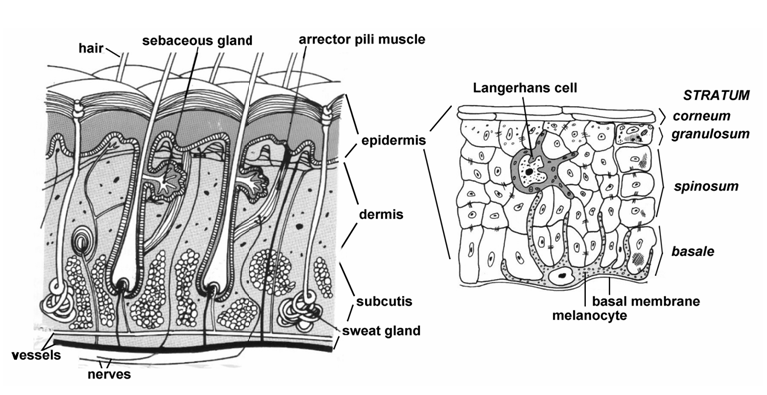
As a major organ, the skin comprises 15-20% of the body’s weight. It is multi-functional, acting as a barrier that keeps internal fluids in and external fluids out. It participates in temperature regulation through constriction and dilation of blood vessels, providing insulation, and allowing for evaporation of sweat. It protects from potentially harmful ultraviolet light through the production of keratin and melanin. It provides sensory input as nerve endings at the junction between the outer and inner layers of the skin (epidermis and dermis) send messages to the brain about the external environment. In addition, the skin is an organ of metabolism, providing, for example, Vitamin D synthesis and lipid storage.1
The three layers of the skin
The skin consists of three layers: the outermost epidermis (“epi:”Greek, over or upon), its underlying dermis, and the tissue beneath the dermis, the hypodermis or subcutis. The epidermis originates embryologically from ectoderm and the dermis from mesoderm.
The epidermis
The epidermis is the thinnest, outermost layer of the skin, measuring less than 0.1 mm on the eyelids to nearly 1 mm on the palms and soles.2 The epidermis is made up of stratified, squamous epithelial cells. Epithelium, (the tissue that forms glands and lines both the cavities and surfaces of all structures of the body), performs a myriad of functions: secretion, absorption, protection, transcellular transport, and detection of sensation. Epithelial tissue that is only one cell thick is known as simple epithelium. Two or more layers of thickness are called stratified epithelium. In some cases, when cells are not even in height, the layers are called pseudostratified epithelium. Cells can be “squamous:” with the appearance of thin, flat plates with horizontally flattened, elliptical nuclei, “cuboidal,” (appearing square in cross section with spherical nuclei,) or “columnar,” elongated and column-shaped with elongated nuclei.
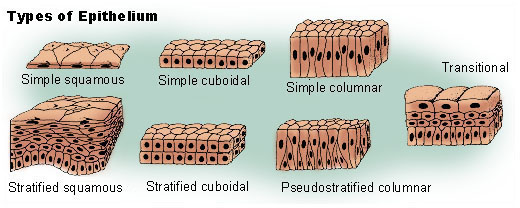
Stratified epithelium can be either “keratinized” or transitional. Transitional epithelium is found in tissue that stretches. It changes its shape from stratified cuboidal to stratified squamous depending on distention. Transitional epithelium is mostly found in the urinary structures. The term “transitional epithelium” is sometimes used by vulvar dermatologists to refer to the skin of the vulva (labia minora and vestibule) as it transitions from keratinized to mucosal (non-keratinized) epithelium.
The endocervix is made up of simple columnar cells, the ectocervix is made up of non-keratinized, stratified squamous cells, as is the vagina and vestibule. The outer structures of the vulva are made up of keratinized, stratified squamous cells.
The epidermis contains a barrier zone between the living and non-living keratinocytes in the outer third of the epidermis that restricts fluid as well as the passage of toxic or infectious substances. It also contains cells (melanin and keratin) that reduce penetration of ultraviolet light.
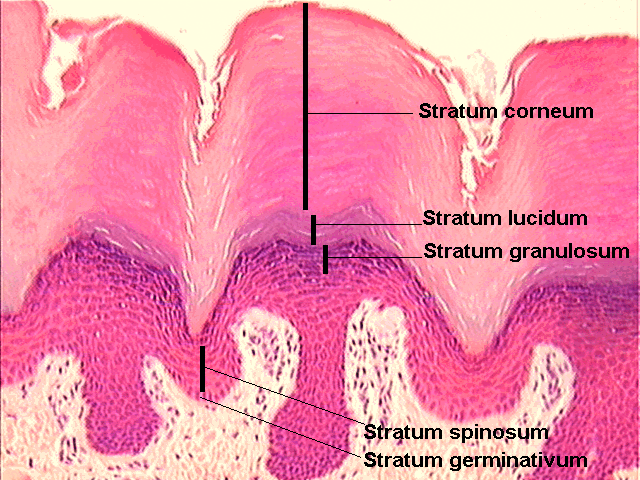
The epidermis does not contain blood vessels but, rather, is nourished by diffusion from blood vessels found in the dermis and hypodermis. The epidermis is subdivided into the following strata (beginning with the uppermost layer):
Epidermis layers
- stratum corneum
- stratum granulosum (granular cell layer)
- stratum spinosum (prickle cell layer)
- stratum basale (basal cell layer)
- basement membrane
The stratum basale and stratum spinosum layers are sometimes known, together, as the Malpighian layer. On the palms of the hands and the soles of the feet, there is another, specialized layer beneath the stratum corneum called the stratum lucidum which is not relevant to a discussion of the vulva.
The epidermal/dermal junction: the basement membrane
The basement membrane separates the ectodermally-derived epidermis from the mesodermally-derived dermis. The two different tissue types are attached to each other through an irregular surface on the bottom of the epidermis (the rete ridges) that fits together with the irregular, papillae-like top surface of the underlying dermis (papillary layer). In cross-section under microscopy, this layer is seen to undulate. Pathology reports on vulvar biopsies often refer to the appearance of the rete ridges or the dermal papillae when explaining diagnoses. For instance, psoriasis causes an increased number and height of dermal papillae.3 Lichen sclerosus causes a decreased number and size of dermal papillae.4
 neuromedia.neurobio.ucla.edu
neuromedia.neurobio.ucla.edu
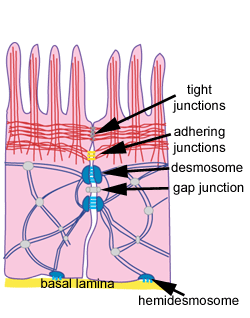
The tissue of the basement membrane includes the plasma membrane of the epidermal basal cells, along with their hemidesmosomes, an electron lucent layer called the lamina lucida, an electron dense band called the basal lamina, and anchoring connective tissue fibrils protruding up from the dermis below.
The four cell types of the epidermis: keratinocytes, melanocytes, Langerhans cells, and Merkel cells
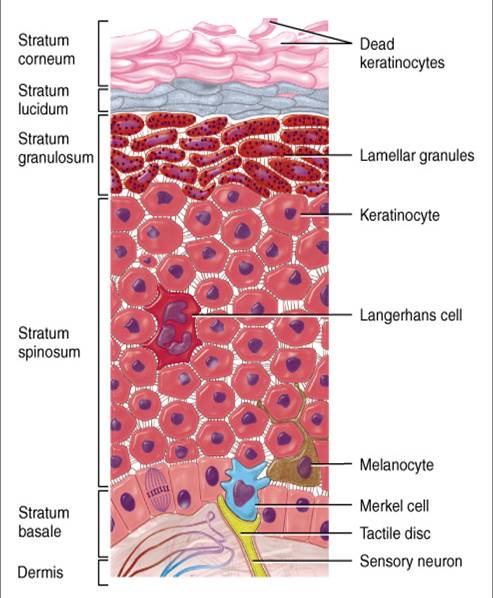
Keratinocytes are the main cell types of the epidermis, comprising 85-95% of all cell types. They are formed through mitosis and proliferation at the basal or germinative layer (stratum basale) where they are called basal cells. A basal cell divides and the daughter cell moves up the layers, maturing, changing shape and composition, then dying due to isolation from its blood source. The remaining cell goes on to divide again. The basal cells are attached to each other and to the underlying basement membrane in a series of junction points known as desmosomes, which also are in a state of flux. Desmosomes are constantly reabsorbed and rebuilt as cells move by one another.5 Through these desmosomes and other connecting points known as gap junctions, a constant 200-A distance is kept between adjacent cells, allowing diffusion of nutrients to the cells as they move outward. These structures perform the work of providing intercellular channels for cell-to-cell communication and strengthening of the cellular structure.
This microsystem within the epithelium provides rapid response to environmental conditions and plays a part in skin healing, for which the keratinocytes play a large role. Not only do they provide the horny layer of primary protection, they also produce a large number of cytokines and can be induced by gamma interferon to express HLA-DR. They release large amounts of interleukin-1 after injury and this initiates immune and inflammatory cascades. They also self regulate epidermal proliferation and differentiation.6
As the cells migrate outward, the desmosomes become visible as prickles or spines (hence the name prickle or spinous layer). In the condition pemphigus, auto-antibodies to the proteins found in desmosomes cause detachment of keratinocytes from one another, causing epidermal blister formation.7 In the prickle layer, keratin is made.
In the process known as keratinization, which takes place over about 27 days, the cytoplasm of the upwardly mobile cells is released and the protein keratin is inserted. In the outermost portion of the spinous layer, keratinocytes change: two types of granules appear in the cytoplasm and the cells flatten. Keratohyalin granules contain the protein filaggrin, which assists in forming the aggregates of keratin fibrils (like steel rod-reinforced concrete). Lamellar granules (also called membrane-coating granules or Odland bodies) attach to the cytoplasmic membrane and release lipids into the intercellular space as flattened liposomes (mortar between bricks of keratinocytes); they are responsible for both water retention in the outer epidermis and for formation of the water-impervious barrier zone. In the outer part of the granular layer, the flattened keratinocytes lose visible evidence of the keratohyalin granules and, as they die, lose their nuclei.8
The keratinocytes eventually reach the “horny layer,” the corneum (top stratum) as 25-30 layers of flattened, denucleated, dead cells filled with keratin which, finally, after 4 weeks, slough off (desquamate); the epidermis, therefore, maintains the same thickness under normal conditions. The whole process takes about 48 days, longer in older people (30-60 days range).9 The keratinized layer of skin is responsible for keeping water in the body and keeping harmful chemicals and pathogens out, making skin a natural barrier to infection.
Melanocytes, making up 5-10% of cells in the epidermis, migrate during the first three months of embryological development from the neural crest and are found in the deepest layer of the epidermis, the basal layer, in a ratio of 1 melanocyte to 30-40 keratinocytes. They produce melanin, the pigment that gives the skin, eyes, and hair their color and protects us from solar irradiation. Melanin is transferred (in the form of melanosomes, pigment granules) to the cytoplasm of the surrounding keratinocytes by means of the cells’ dendritic processes. Differences in skin color are not due to the number of melanocytes, but rather to the numbers and size of melanosomes and their hormonally-regulated cellular activity, which is genetically determined.
Skin darkening occurs via two pathways: 1) immediate photo-oxidation of pre-formed melanin and 2) delayed new melanin production via tanning. Increased melanin production is protective, preventing mutational DNA damage. Darkening of the skin also occurs with endocrine changes that cause increased MSH or ACTH and through inflammation (post-inflammatory hyperpigmentation).10
Groups of melanocytes form nevi (moles). Nevus cells are pigment-producing cells derived from melanocytes. They lack dendritic processes and occur in nests or clusters (nevi). Collections of these nests are called compound nevi; lesions containing nests of nevus cells only within the dermis are called dermal nevi. Nevi are present during early fetal life and are extremely common. They continue to develop after birth, appearing anywhere on the skin, apparently influenced by sun exposure to some extent. They sometimes display immune-response changes. Some nevi have the potential to develop into malignancies.
Langerhans cells (1-5% of the cells in the epidermis) are derived from bone marrow and are found in the Malpighian layer, as well as in thymic and mucosal tissues and in lymph nodes. Langerhans cells are also dendritic cells, but they do not produce pigment; they function as one of the body’s immunological defense systems. They detect antigens which they transport to the lymph nodes in the dermis, where lymphocytes take over the defense process. The processed antigens are presented to the immunocompetent helper T cells which trigger the inflammatory reaction of allergic contact dermatitis.
Langerhans cells are susceptible to deactivation or destruction by UV light which may cause immune tolerance, leading to skin malignancy. They are also easily infected by retroviruses.
Merkel cells: The embryological origin of Merkel cells is unknown. They make up 6-10% of the cells in the epidermis and are located in the stratum basale between the keratinocytes where they remain in contact with nerve endings. They serve as mechanoreceptors and are involved in the function of light touch and shape detection. Merkel cells may undergo uncontrolled proliferation to form aggressive, though rare, skin malignancies.
The dermis
The dermis, (the layer of skin beneath the epidermis and the basement membrane) mostly consists of connective tissue and cushions the body from stress and strain. The dermis is structurally divided into two areas: a superficial area adjacent to the epidermis and part of the epidermal/dermal junction, called the papillary region, and a deep, thicker area known as the reticular region. The papillary region contains fingerlike projections of loose connective tissue (papillae) that extend up toward the epidermis, strengthening the connection between the two layers of skin. The reticular region lies deep down and is usually much thicker. It is composed of dense, irregular connective tissue with elastic and reticular fibers that provide the strength and elasticity of the skin.
Nerve supply to the dermis
Mechanoreceptors in the dermis provide the sense of touch and heat. Non-myelinated sensory nerve fibers are primarily responsible for itching and light pain, and myelinated fibers are principally responsible for deep pain, pressure, and temperature.11
Motor nerves are autonomic and responsible for the function of eccrine sweat glands, for blood vessel flow rates, and for activity of the tiny muscles of the hair follicles.
Blood supply to the dermis
Arteries in the subcutaneous tissue, along with their venous counterparts, narrow as they course through the dermis toward the epidermis. There is a deep plexus of arterioles just above the subcutaneous fat that supplies the sweat glands and hair papillae and a superficial plexus in the papillary dermis whose arterioles end in capillary loops higher in the papillary dermis.12 The network of blood vessels diffuses nutrients to the upper dermis and the epidermis. With dilation and contraction, blood vessels help to regulate temperature in the body. They also carry leukocytes to the skin where they participate in immunologic and inflammatory responses.13
Cutaneous lymphatics
Afferent lymphatics begin as blind-ended capillaries in the dermal papilla and pass to a superficial lymphatic plexus in the papillary dermis, along with two deeper plexuses, one extending down into the superficial fascia.14
Cell types in the dermis
A predominant cell type in the dermis is the fibroblast. Fibroblasts synthesize the extracellular matrix and collagen that characterize the dermis. Not only do they create the structural framework that ensures strength and flexibility in the skin, they also play a crucial role in wound healing.
Other cell types in the dermis include melanocytes and Merkel cells (discussed above), mast cells, macrophages, and other cells of the immune system.
Mast cells are scattered throughout the dermis and contain many granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in wound healing and defense against pathogens. Mast cells play a key role in the inflammatory process and in allergic reactions.15
Mast cell activation may also be followed by the synthesis of chemokines and cytokines. Cytokine and chemokine secretion, which occurs hours later, may contribute to chronic inflammation. Biological functions of mast cells appear to include a role in innate immunity, involvement in host defense mechanisms against parasitic infestations, immunomodulation of the immune system, and tissue repair and angiogenesis.
Macrophages (Greek, big eater) are phagocytes: cells that engulf foreign substances, infectious microbes and cancer cells, as well as other types of pathogens, thereby ingesting and destroying them. They play a role in both innate and adaptive immunity and stimulate lymphocytes and other immune cells to do the same. As such, they play a central role in wound healing, both stimulating production of inflammatory cytokines and growth factors and also promoting proliferation of fibroblasts, keratinocytes and endothelial cells to rebuild epithelium and heal blood vessels. Malfunctioning of macrophages in the wound healing process can promote ulcers, keloids and other scars, and chronic wounds.
Skin appendages
Sweat glands, sebaceous and mammary glands, hair and nails–the so-called skin appendages–are derived embryologically from downgrowths of epithelium from the epidermis into the dermis and subcutis.
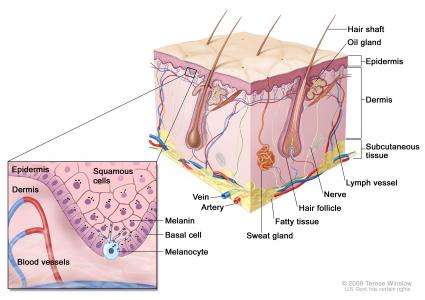
Hair follicles
Hair follicles appear early in embryological life and produce both the follicle and the hair, which is specialized according to location on the body for protective purposes. Hair follicles are found everywhere on the skin except for the palms, the soles, and the mucous membranes. Ectopic hair follicles, mostly presenting with the sebaceous gland component, can appear on some mucous membrane surfaces (e.g. inner aspects of the labia minora) as Fordyce spots: harmless, round, smooth papules containing sebaceous material.
Sebaceous glands
These glands are formed by budding of the epidermally-derived cells of the hair follicle, with egress at the surface of the epidermis, alongside the hair. They produce sebum, an oily substance whose purpose is to prevent excessive drying of the hair and skin. Sebum is odorless but, if broken down by bacteria, can develop an odor. It can accumulate in the form of sebaceous cysts, usually benign, which, if infected, can cause pain. In addition, unique to the genitalia is the formation of smegma, an accumulation of sebum combined with dead epithelial cells. This white, pasty substance can be found in the interlabial sulci and around the clitoris. Usually harmless, bacteria and occlusion of the skin, with associated moisture, can cause irritation in the areas that form smegma.
Apocrine sweat glands
Apocrine sweat glands are distinguished from eccrine sweat glands by their anatomical positions and functions. Like the sebaceous glands, they develop from follicular epithelial cells. Their coiled “roots” are located at the base of the dermis, (partially in the subcutis), but the ducts find egress through the hair follicles. These glands are responsible for the subtle, pheromonal scents that are linked to sexuality and may have had other primordial functions earlier in human evolution. Apocrine sweat glands are inactive until they are stimulated by hormonal changes in puberty; they are activated not for temperature regulation, but in response to emotional or physical stress. In the human, they are located primarily in the axillae, the perianal areas, and on the areolae of the breasts. Glandular breast tissue is derived from modified apocrine glands, and ear wax is produced by apocrine glands, as well. The viscous “sweat” produced by these glands is odorless unless activated upon by skin bacteria.
Eccrine sweat glands
The ducts of eccrine glands find egress directly on the surface of the skin, not through hair follicles. Their most important function is to aid the body in temperature regulation. Sweat glands are present all over the body. Those located on the palms, soles, axillae, and forehead are under both psychological and thermal control, whereas those located elsewhere respond solely to thermal stimuli.16 Eccrine sweat glands are innervated by sympathetic nerve fibers, but physiologic mediation of sweating is due to acetylcholine release. Sweat is composed of isotonic plasma-like fluid in the secretory portion of the gland but changes in composition by the time it reaches the epithelial surface.17 Sweat serves to cool the skin through evaporation and up to 10 liters a day may be produced in some circumstances. In the vulvar area, eccrine glands contribute to dampness and in some individuals, hyperhidrosis. (Annotation A: Patient symptoms and signs).
The hypodermis
The hypodermis lies below the dermis. Its purpose is to attach the skin to underlying bone and muscle as well as to supply it with blood vessels and nerves. It consists of loose connective tissue and elastin. The main cell types are fibroblasts, macrophages, and adipocytes (the hypodermis contains 50% of body fat). Fat in this area serves as padding and insulation for the body. Another name for the hypodermis is the subcutaneous tissue or subcutis.
The vulvovaginal terrain varies topographically, from the hills and valleys of the mons pubis, the labia majora and minora, and the interlabial sulci to the outcroppings of the clitoris and prepuce, and the “caves” of the introitus and vagina, urethra, and vestibular glands.
The vulvovaginal epithelial terrain is as varied as the anatomical. It is the only tissue in the female body that derives from all three embryological layers (ectoderm, endoderm, and mesoderm) in close proximity,18 varying in type from the outermost to the innermost structures, depending on biological function. This crossroads of three different tissue types gives rise to the separate ecosystems of the vulva: the mostly arid climate of the outer structures, the intermediate regions of the inner labia minora and vestibule, and the more jungle or rainforest-like environment of the vagina.19
Each biosphere of the vulva and vagina has its own epithelial and glandular make-up, hormonal responsiveness, neural distribution, immune response, and relationship to other body systems.20 Alterations in the tissue may arise from underlying structures, from epithelial disorders present elsewhere in the body, from non-dermatological diseases, immunological disorders, infectious processes, infestations, hormonal changes, or from irritants such as clothing, personal hygiene products, or treatment attempts.
Understanding the embryological origins and anatomy of the vulvovaginal epithelium helps to prepare the clinician for the differential diagnoses that will be most likely in each particular area.
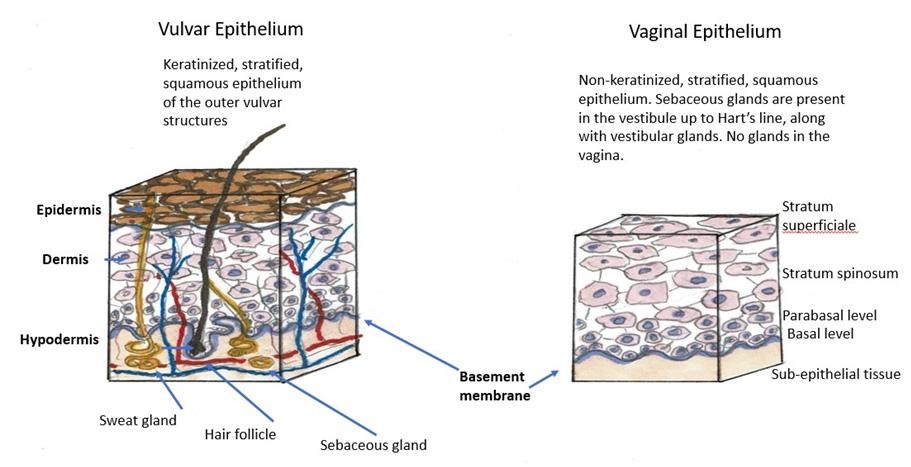
Ectoderm-derived vulvar skin
The skin of the mons pubis, the labia majora and minora, the clitoris, prepuce, and the perineum is derived from the embryonic ectoderm. Similar to the exposed skin of the majority of the body, the outer structures are well-keratinized, and composed of stratified squamous epithelium, providing a rugged cover (meaning of the word vulva) for the tissue. The skin of the outer structures (mons pubis and labia majora) is rugose and sometimes a bit darker in color than the adjacent skin of the inner thigh. Neoplasms of this terrain are related to the sweat glands, sebaceous glands, and hair follicles found here: hidradenitis suppurativa, folliculitis, sebaceous cysts, infestations, as well as the dermatoses. Ectoderm-derived regions prefer to be dry and can develop pathology if flooded by the moisture provided by the numerous eccrine and apocrine glands present. Occluding the area with clothing promotes moisture and friction, provoking problems, as well.
Cutaneous thickness and degree of keratinization characteristic of the mons pubis and labia majora, decrease over the anterior portions of the clitoris and from the outer surface of the labia minora to their inner surfaces.21 The inner majora, interlabial sulci, and minora are semi-arid regions, moisturized by sebum, not as dry as the outer majora.
Although the medial aspects of the labia majora and the entire labia minora are generally considered to exhibit hairless mucous membrane epithelium, the partially keratinized skin here contains several structures, including subtle hair follicles.22 They are subject to pathologies of the pilo-sebaceous unit such as hidradenitis, as well.
Endoderm and mesoderm derived vulvar skin
The mucosa of the vulvar vestibule is the only portion of the female genital tract of endodermal origin, 23 (although the anterior urethral wall and the bladder (outside of the trigone) are also derived of endoderm.) Mucosa is made up of non-keratinized cells that are large, moderately flattened, and contain glycogen granules and pyknotic (shrunken) nuclei. The strata of the epidermis are less clearly demarcated, containing loosely packed, polyhedral cells.24 Langerhans cells are present in this tissue.
Vestibular skin
The vagina, hymenal membrane, bladder trigone, and posterior urethral wall are of mesodermal origin. The nonkeratinized squamous epithelium in this area is responsive to ovarian steroid hormone cycling.25 Annotations O: The vaginal epithelium, Annotation P: Vaginal secretions, pH, microscopy and cultures, and Annotation Q, The hymenal ring and bimanual exam for discussion of the mesodermal tissue.
Medial to Hart’s line, both of these mucosal areas are damp, with variable wetness from the greater and lesser glands of the vestibule and from transudation and cervical secretions in the vagina. Occlusion of the skin with clothing can exacerbate problems in these areas. Loss of the moist environment from disease such as eczema or hormonal disruption leads to irritation, dryness, and pain.
Significance of the epithelial barrier
The vulvovaginal epithelium functions, like all epithelium, as a protective barrier for the terrain. Loss of the integrity of the epithelial barrier occurs with many vulvovaginal disorders including atrophy, allergic or irritant contact dermatitis, and erosive, ulcerative, and bullous disease. When the terrain is denuded, the epithelial protection is lost. The patient experiences irritation, burning, intolerance to topical treatments, and eventually, even to water. Therapy will not be possible without initial treatment to restore the barrier by hydration with plain water, if tolerated, or physiologic saline baths followed by sealing in the moisture with petrolatum.
Age and hormone-related changes in the vulvar skin
The vulvar skin is thickest in the reproductive years, but the impact of menstrual cycle on changes in vulvovaginal epithelium has not been well studied. At mid-cycle, there are subtle changes in the surface cells (changing from orthokeratotic (lacking nuclei) to parakeratotic (with degenerated nuclei) which are related to hormonal shifts but do not make a known difference clinically.26 Vulvar skin has a higher concentration of epidermal androgen receptors than skin at non-genital sites. At puberty, androgens direct the maturation of vulvar sebaceous glands and their hair follicles.27 Whereas the vaginal epithelium contains a high number of estrogen receptors and is responsive to ovarian hormone cycling, estrogen receptors decrease in prevalence moving outward to the vulva, with lowest levels on keratinized skin. Progesterone receptors are not found on outer vulvar skin; they are restricted to the epithelium as it transitions from the inner aspect of the labia minora to the nonkeratinized epithelium of the vagina and vulvar vestibule.28
The vestibular skin of reproductive-age women has not been well studied in relation to particular hormonal effects. One recent study, however, looked at the potential morphological effects of oral contraceptives on vestibular tissue. On review of the biopsies taken at different times in the cycles from the vestibules of women on the pill and the control group who were having natural menstrual cycles, the researchers found changes in dermal papillae that did suggest influence of hormones. In the follicular phase, vestibular dermal papillae of women on the OCP were more shallow and sparse compared with those of women having natural cycles. In the luteal phase, vestibular dermal papillae of women having natural cycles but not those of women on the pill were shallower and sparser.29 The clinical significance of these findings is unknown.
In pregnancy, vulvar connective tissue relaxes and increased progesterone may cause vulvar varicosities. The labia majora enlarge and may darken in color. One recent study showed that vulvovaginal complaints of itching and burning increased in pregnancy but decreased postpartum. Dyspareunia also increased in pregnancy but continued postpartum.30 The effects of lactation were not examined in this study, but lactation clearly affects vaginal and vestibular mucosa. (Annotation O).
Postmenopausal women exhibit sparser pubic hair as time goes by and there is less subcutaneous fat in the labia majora. The labia minora may thin. Hydration, elasticity, and permeability in the skin do not change in older women, but exposure to urine due to incontinence can cause problems.31 Exposure to urinary moisture, especially with occlusion from clothing or panty protectors, makes the skin more susceptible to friction damage. Urinary ammonia elevates local pH, which alters skin barrier function and activates fecal enzymes. Fecal enzymes further compromise skin integrity and increase susceptibility to microbial infection. The reduced mobility of some women as they age exacerbates these risks.32
Vaginal and vestibular atrophy in postmenopausal women (and postpartum women who are lactating) is well documented (Annotation O, The vaginal epithelium). The hormonally mediated changes of the menopause can cause pain and bleeding with penetrative sex.
Microbial factors
The microbial flora of the labia majora include a high density of microorganisms common for intertriginous skin such as gram-negative rods, and also organisms unique to the vulva and probably related to urethral and vaginal flora, such as the nonpathogenic neisseria, lactobacilli, and Gardnerella vaginalis. In addition, the skin of the labia majora is a preferred site of Staphylococcus aureus, which can be of clinical and epidemiological relevance.33 Microorganisms like Staphylococcus epidermidis colonize the vulvar skin surface. The density of skin flora depends on the region of the skin. The disinfected skin surface gets re-colonized from bacteria residing in the deeper areas of the hair follicle, gut and urogenital openings. Staphylococcus aureus, coagulase-negative staphylococci, streptococci, diptheroids, lactobacilli, and yeasts have all been found on the labia majora in larger concentrations than on the forearm in healthy women.34 These organisms rarely cause infection unless there is an underlying insult to the skin that causes fissures, erosions, or ulcers. In some cases, (e.g. Candida infection) conditions which affect the vagina also affect the vulvar skin and vice versa (e.g. lichen planus). Methicillin resistant staph aureus (MRSA) is still rare in the vulvar area, but is on the rise. (Methicillin resistant staph aureus). (Microbes affecting the vagina have been reviewed in Annotation O, The vaginal epithelium, and Annotation P, Vaginal secretions, pH, microscopy and cultures.)
Immune cell populations of the vulva and vagina
Langerhans cells predominate in the vulva whereas lymphocytes predominate in the vagina.35 It has been suggested that the Langerhans cells have been the primary target of vaginally transmitted HIV infection.36 Interestingly, different regions of the vulva and vagina show different responses to antigen application. Experimental antigen application to the vulvar skin can result in sensitization and allergic contact dermatitis whereas antigen application to nonkeratinized mucosa may induce tolerance.37
Mast cells play an important role in the inflammatory process in the vulvovaginal environment. Histamine, cytokines, heparin and other inflammatory mediators are released when the mast cell is activated by an allergen or traumatic event, often after reacting with IgE antibodies first. The release of histamine and other biochemical substances can cause swelling, redness, itching and pain in allergic reactions, a healing mechanism that causes the host temporary discomfort.
Cytokines are also mediators of immune responses. They are hormone-like glycoproteins that coordinate immune responses and enable cells to communicate with each other. They are delivered locally or through the circulation and are produced by immune cells and by non-immune cells, e.g. fibroblasts and endothelial cells. Cytokine response has been studied by researchers trying to understand vulvar pain syndromes.
Immune response
Understanding a few elementary mechanisms of the immune system can be helpful in understanding some vulvar problems. Immune responses are either innate or adaptive. Innate responses to microorganisms are immediate and rapid. We are born with them. On introduction of an antigen, pattern recognition receptors (PRRs) summon neutrophils and macrophages, communicating through cytokines. The response creates redness, swelling, pain, or itching, simultaneously providing defense and causing temporary damage to the host. In addition, innate responses are “amnestic,” that is, there is no cellular memory of the event (i.e. there is “amnesia”) and exposure to the same antigen causes the same response every time.38 The same cells participate in maintaining homeostasis in the host, regulating and repairing inflammation they themselves initiated.
Adaptive responses are either humoral or cell mediated. They are slower in their responses than innate responses. Humoral responses depend on antibody production by B cells and plasma cells. Cell mediated immunity involves the interactions of helper and cytotoxic T cells with various target cells.39 Adaptive immune responses are characterized by a slower response time and specific antigen recognition, as well as the ability of the body to remember the antigen readily. (See the following related Atlas of Vulvar Disorders items: Contact dermatitis: allergic and irritant, Eczematous dermatitis, and Lichen simplex chronicus).
Changes in the vulvar skin that lead to complaints can arise from many sources:
Table H-1: Origins of vulvovaginal skin conditions and associated annotations
| Anatomical variations (F) |
| Normal physiological variants (Atlas of Vulvar Disorders) |
| Congenital variants (F) (N) (Q) |
| Autoimmune conditions (B) |
| Irritant or allergic contact dermatitis (Atlas of Vulvar Disorders) |
| Eczematous dermatitis (Atlas of Vulvar Disorders) |
| Dermatoses (skin diseases) (Atlas of Vulvar Disorders) |
| Immunosuppression (B) |
| Fixed drug reactions (Atlas of Vulvar Disorders) |
| Systemic disease (B) (Atlas of Vulvar Disorders) |
| Infectious conditions (bacterial, fungal, protozoal, viral) (P) (Atlas of Vulvar Disorders) |
| Low estrogen states (O) |
| Pre-cancer or cancer |
| Neuropathic conditions (K) |
| Trauma (Atlas of Vulvar Disorders) |
| Infestations (Atlas of Vulvar Disorders) |
| Factitial causes (self-inflicted) (Atlas of Vulvar Disorders) |
Anatomical variations, normal physiological variants, congenital variants
Not all changes in the vulvar skin are “bad.” Some benign lesions appear and cause concern to women, who worry about lumps and bumps even if they are asymptomatic. Examples of benign lesions are acrochorda (skin tags), Fordyce spots (small collections of sebaceous material), or vulvar or vestibular papillae, (tiny protrusions, symmetrically arranged, with rounded tips, usually seen around the vestibule). Anatomical variations have been reviewed in Annotation F: The vulvar architecture, in Annotation O: The vaginal epithelium, and in Annotation Q: Hymenal ring and bimanual exam).
Autoimmune conditions, allergic or irritant contact dermatitis, eczematous dermatitis, dermatoses, fixed drug reactions, immunosuppression
Many skin conditions have autoimmune components that make individuals more likely to have specific changes in the skin. These include the various types of dermatitis (Atlas of Vulvar Disorders for Contact Dermatitis: irritant and allergic, Eczematous dermatitis, Lichen simplex chronicus). The dermatoses (Lichen sclerosus, lichen planus, bullous pemphigoid, pemphigus vulgaris, cicatricial pemphigoid, erythema multiforme, aphthosis, toxic epidermal necrolysis, psoriasis) that affect the vulva are also thought to have autoimmune components. The definition of the word dermatosis is: “A skin disease, especially one that is not accompanied by inflammation.” It is the case, however, that many vulvar diseases are inflammatory and are called dermatoses by dermatologists.
In both autoimmune and inflammatory diseases, the condition arises through aberrant reactions of the human adaptive or innate immune systems. In autoimmunity, the patient’s immune system is activated against the body’s own proteins. In inflammatory diseases, it is the overreaction of the immune system, and its subsequent downstream signaling, which causes problems.
Fixed drug reactions are probably an allergic (hypersensitivity) reaction with an autoimmune component, as well.
Immunosuppression can alter the immune response and make women more susceptible to certain infections.
Systemic diseases
Certain systemic diseases are known to be related to vulvovaginal disorders: Crohn disease, Behçet disease, and Sjögren disease are examples. Both atopy and hypothyroidism occur in many of our patients who have vulvar dermatoses. All of these conditions have autoimmune components, as well.
Infectious conditions
Candidiasis, herpes simplex, herpes zoster, impetigo, streptococcal cellulitis, chancroid, and lymphogranuloma venereum are known to cause vulvar symptoms and changes in the skin. (Annotation P)
Low estrogen states
Atrophy related to the postpartum, lactation, or post-menopausal states will cause vulvar symptomatology. (Annotation O).
Pre-cancer or cancer
Vulvar intraepithelial neoplasia (VIN), squamous cell carcinoma, malignant melanoma, basal cell carcinoma, and Paget disease, may all potentially affect the vulvar skin and are covered in the Atlas of Vulvar Disorders.
Neuropathic conditions
Pudendal neuralgia and vulvar pain and provoked and unprovoked vulvodynia are covered in Annotation K. The patient reacts to light touch of the vulvar skin with symptoms of pain, but a specific skin condition causing the pain has yet to be found.
Trauma, infestations, factitial causes of vulvar complaint
These are covered in the Atlas of Vulvar Disorders. Factitial causes are those that are self-inflicted by the patient. (prurigo nodularis and trauma/self inflicted)
The job of the careful and caring clinician is to thoroughly understand what normal anatomy and skin look like in the vulvar area, including benign changes, so that the history can be put together with the exam in such a way that clear answers can be given to the patient.
We have worked out a simple formula for systematically inspecting the vulva. This approach involves methodically scanning the anatomical structures and then the skin itself. This deep consideration of the tissue requires at least three different visual sweeps: one for the anatomical structures, one for the skin, and a third for pain mapping.
Is the anatomy normal?
If we return to the concept of the various terrains of the vulva, the “hills and valleys” of the anatomy are checked for normalcy. Just as we expect to see two eyes, a nose, and a mouth on each face, we expect to see specific structures in the vulva, as well. (Annotation F: The vulvar architecture).
Is the skin normal?
Then, the skin is examined, while asking ourselves the following question: Inspecting from the outermost to the innermost parts of the vulva, is the skin normal in color, texture and integrity?
It is almost impossible to inspect the skin without touching the woman so that the interlabial sulci are checked, the prepuce lifted, the folds of the labia minora visualized on both sides, and the vestibule gently exposed. When a woman has come in expressly for vulvovaginal exam, this careful inspection is usually appreciated. The exam should also be done in the asymptomatic woman, however, to identify problems of which she is unaware. (Example: lichen sclerosus can be asymptomatic while causing extensive loss of normal vulvar anatomy.) In this case, the clinician may feel more comfortable telling the woman that he or she is checking the skin to make sure everything looks normal: “I’m just taking a look to make sure everything looks OK. Are you having any problems in the vulvar area?” In all cases, permission to begin touching must be attained (Annotation D: Patient tolerance for genital exam).
Touching will also enable the clinician to begin symptom mapping. (Annotation I: Symptom and pain mapping and the Q-tip test.) Although the exam is done fairly quickly, with each visual and tactile sweep of the terrain, all of the questions need to be asked and answered, as follows. (Annotation E: The detailed vulvar exam).
Is the skin color normal?
One expects that the color of the vulvar skin will “match” the rest of the individual’s coloring. Redheads and blonds may have ruddy, darker pink or reddish skin with base tones of beige in the vulvar area;

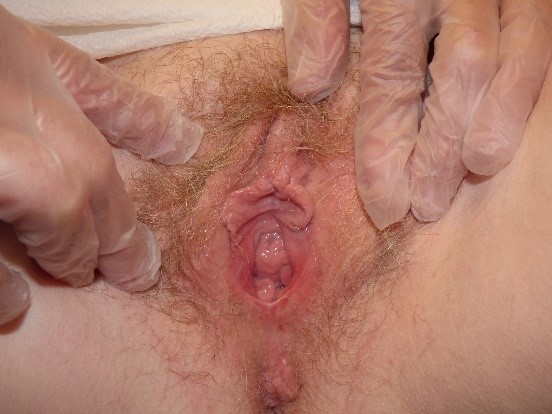
dark-skinned people have less rosy skin with darker areas of normal pigmentation in the labio-crural folds and the labia majora; they may also have increased pigmentation along the edges of the labia minora.
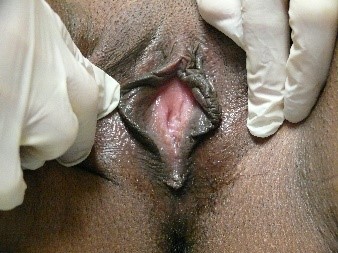
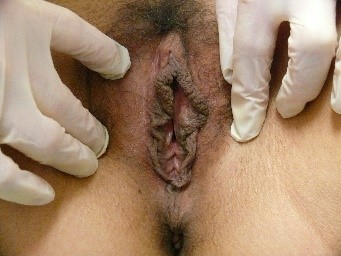
The modified mucous membranes of the vulva are pink in varying degrees. It is useful to take into account the patient’s general coloring and the color of the adjacent tissue (e.g. the inner thigh) for comparison to determine whether the vulvar skin color is normal.
Color is also an important clue when it comes to any lesions on the skin, whether these are benign or related to disorder or disease. Problems in the vulvar skin can present as skin-colored, white, red, pink, blue, purple, yellow, greenish, brown, and black. See the Atlas of Vulvar Disorders for elucidation on various colors of lesions within Morphological Characteristics. (Atlas)
Erythema
Erythema can be understood as excessive redness in comparison to the rest of the patient’s own skin. In the research setting, instruments have been developed to measure subtle differences in skin reflectance, but an experienced eye is thought to be equally effective.40 Erythema is an important clue to problematic vulvovaginal conditions but must be accurately identified to be of use in diagnosis. As light-skinned women often appear pink or red in the vulvar area,
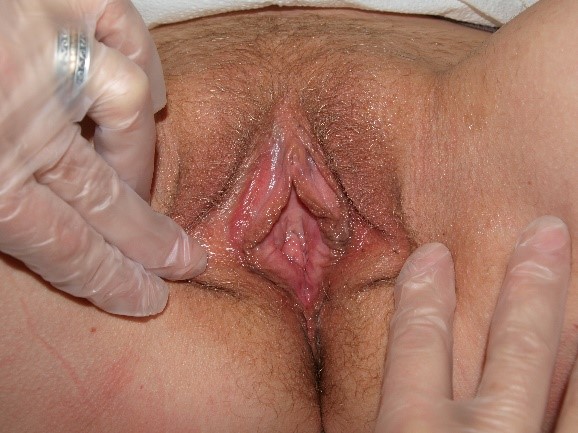
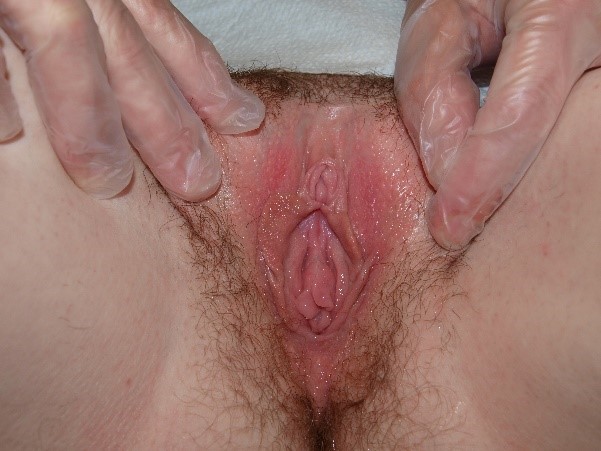

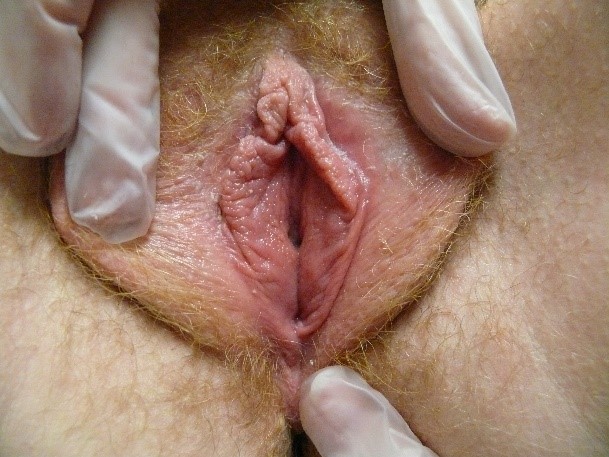
the patient’s history and the mapping of her symptoms (Annotation I: Pain and symptom mapping and the Q-tip test) will clarify whether or not her redness is significant. Erythema in darker skinned people is more subtle and is sometimes masked by darker pigmentation.
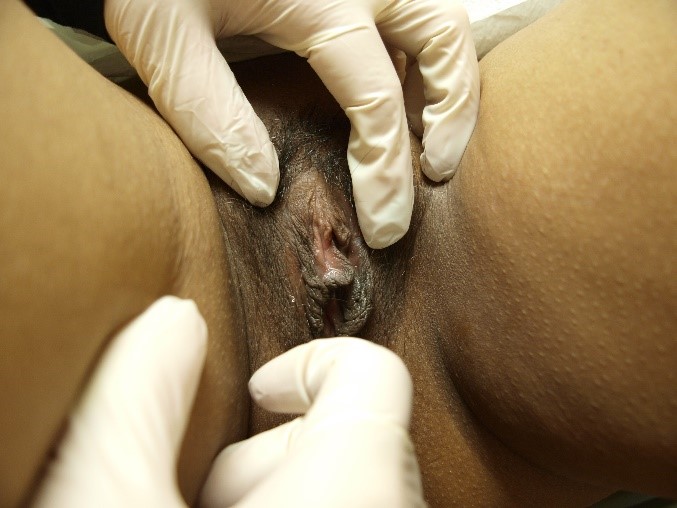
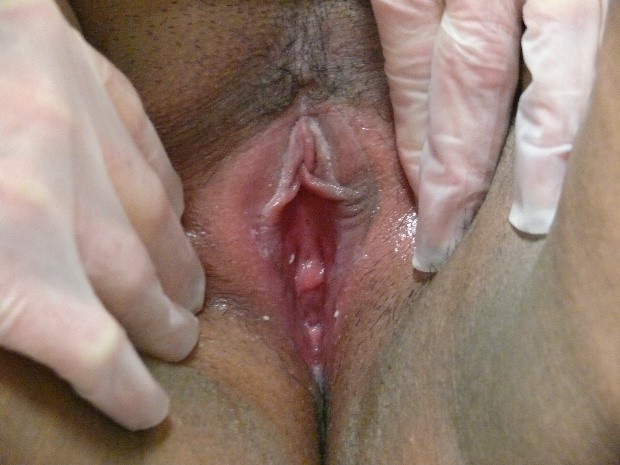
Biopsy (Annotation G: Vulvar biopsy) may be necessary and, in the case of erythema, Candida must always be considered.
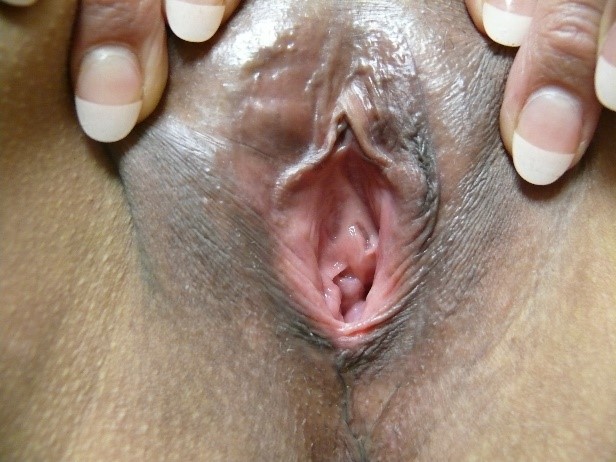
Is the skin texture normal?
Texture refers to surface quality, especially roughness versus smoothness. Normal variations in texture correlate with function of the tissue. It is normal for the hair-bearing (non-glabrous) skin of the labia majora to be rugose or slightly wrinkled in texture and the more keratinized epithelium of this area to be thicker than the less keratinized skin of the labia minora.
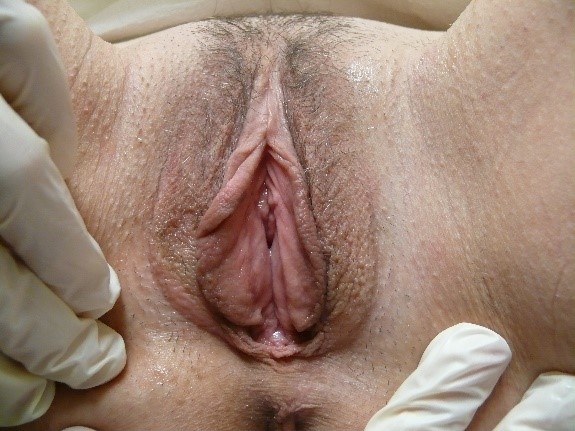
Keratinized skin contains adnexal structures such as sweat glands, sebaceous glands and hair follicles that may give rise to specific disorders, as reviewed above. Transitional epithelium on the inner aspects of the labia minora, crossing Hart’s line to the vestibule is usually quite smooth, although there can be tiny, normal papillae present.
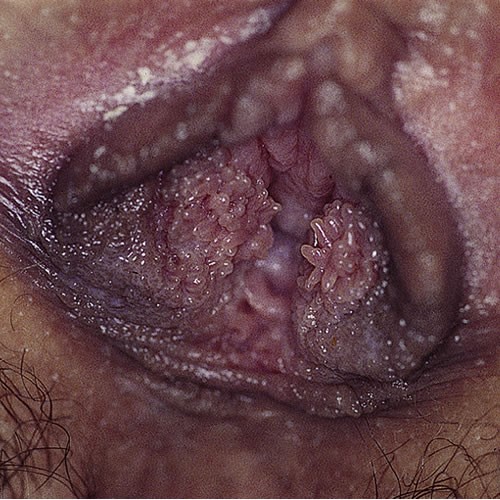
In addition, attention to texture can alert the clinician to look for variations in height of lesions. Example: is the pigmented lesion a macule or a patch
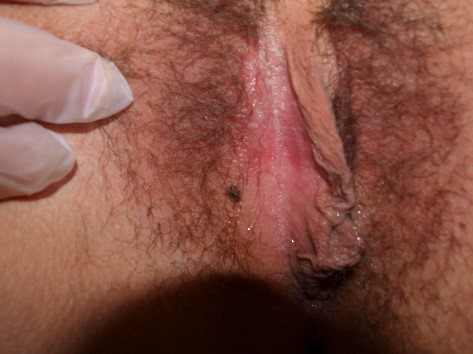
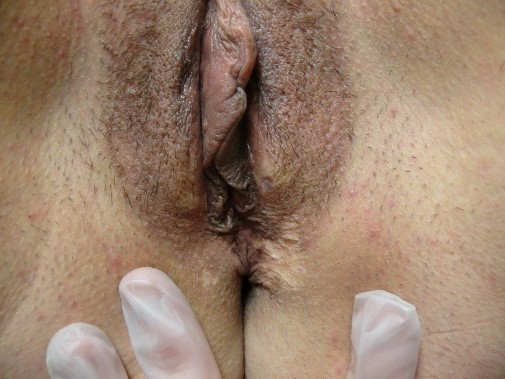
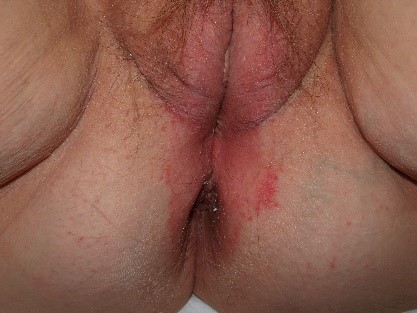
which have no elevation of texture, or a papule or plaque which do.
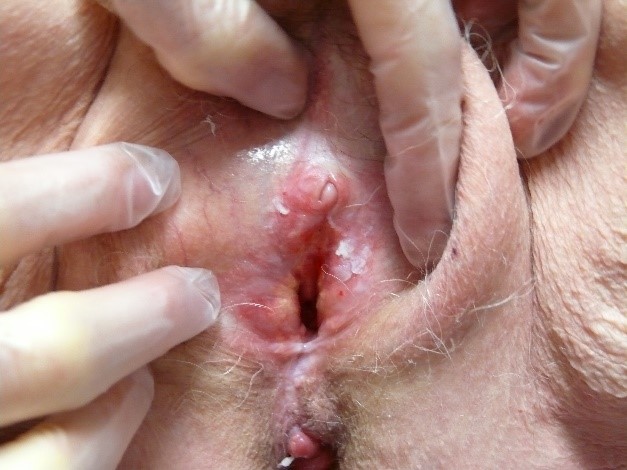
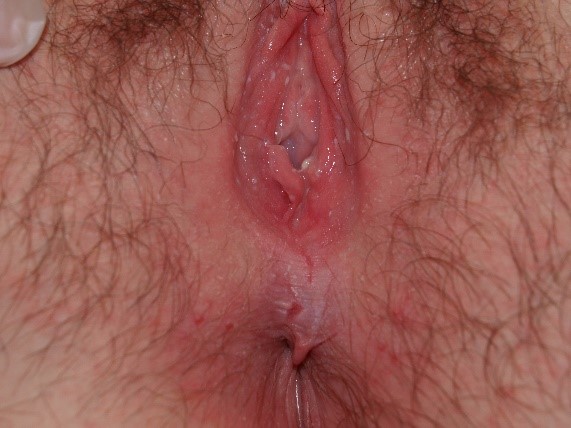
Abnormalities of skin texture
Surface texture can be altered by visually perceptible thickening of the skin known as lichenification (Lichenification in Dermatological Terminology) where the surface may be slightly rough or slightly raised and contain exaggerated skin markings and, sometimes, fine scale. Lichenification occurs as a callus-like response due to chronic rubbing, and is characteristically seen in the related conditions of atopic dermatitis, eczematous dermatitis, and lichen simplex chronicus.

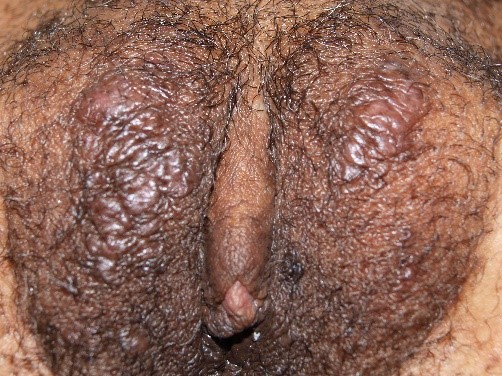
Texture may be altered by “crinkling.” In the case of lichen sclerosus, where skin is expected to be smooth, it can look like cigarette paper,
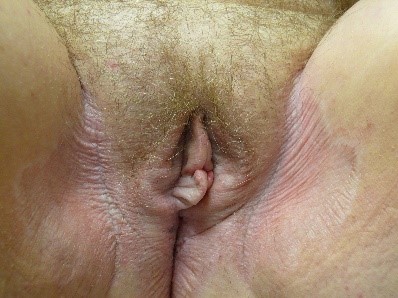
or it may be thickened, whitened, without lichenification.
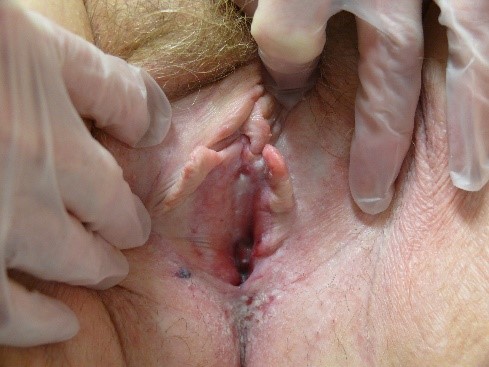
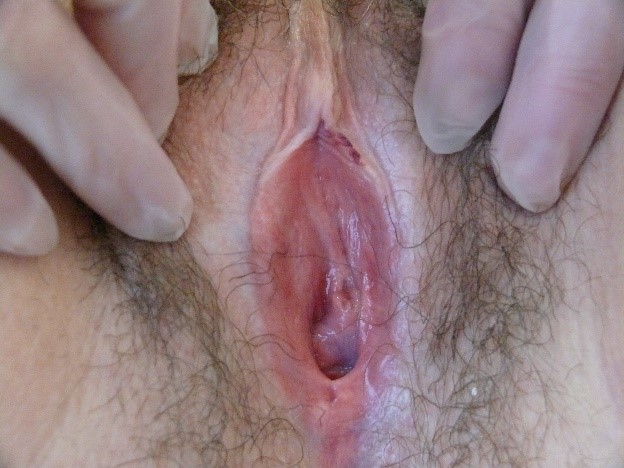
Crusting and scaling can also occur. Nodules, papules, or plaques may be present to interrupt expected surface texture.
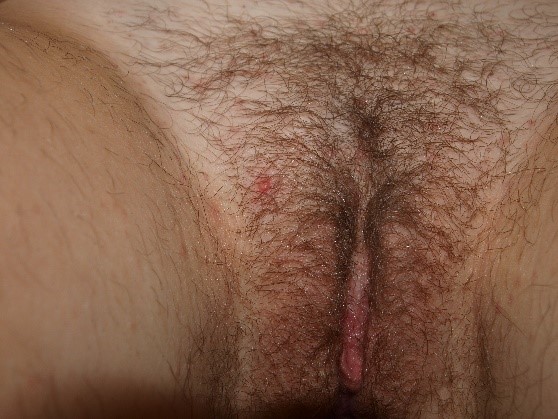
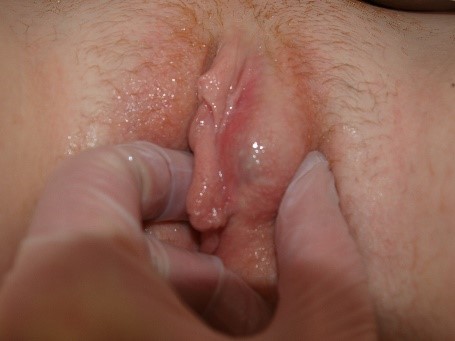
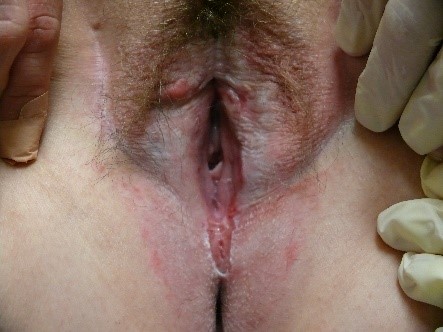
Vesicles (blisters) or pustules may appear
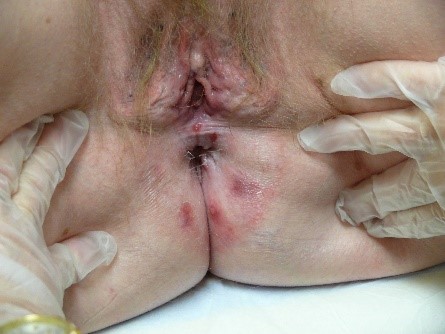
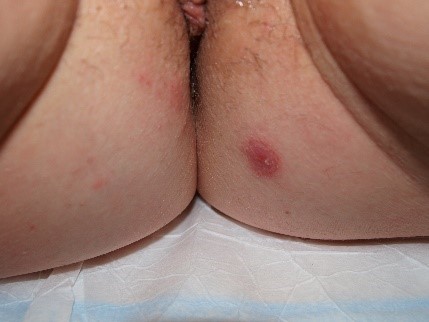
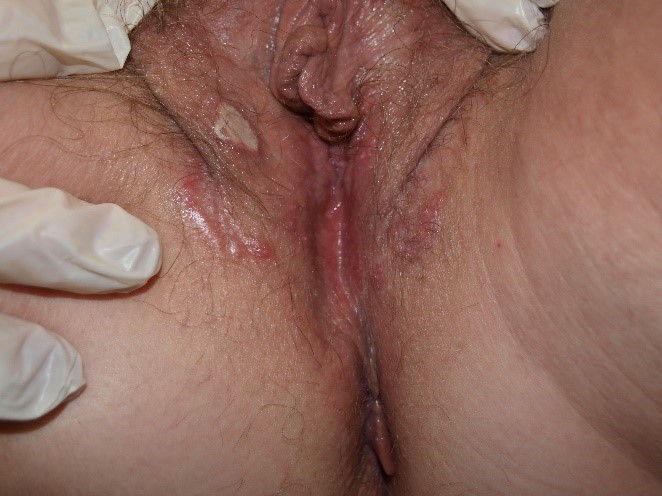

and then transition into surface disruption (alterations in “integrity of the skin”) in the form of erosions or ulcers. The edges of these disruptions in the integrity of the skin may have either a raised
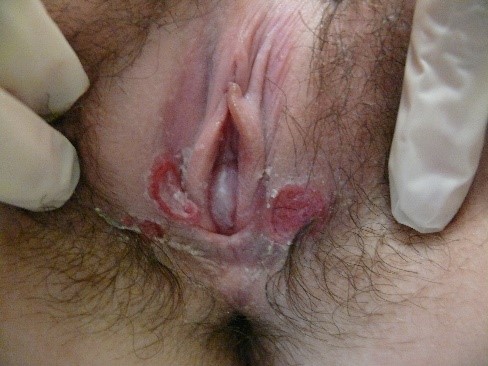
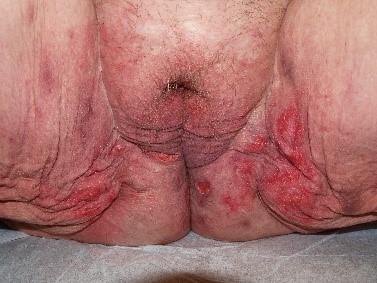
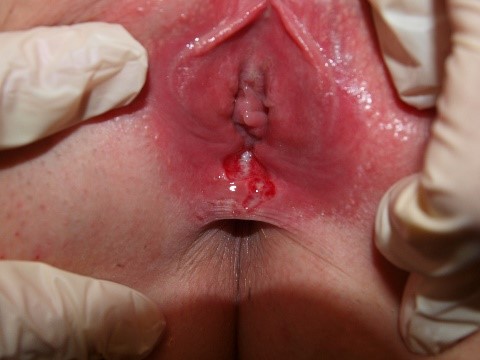
or an undermined appearance that can contribute to diagnosis.
Edema may be caused by inflammation, allergic reactions, infection and cellulitis, lymphatic obstruction, or systemic disease. Edema changes surface texture as the skin accumulates fluid in the dermis or subcutaneous tissue and swells. Edematous changes can be skin colored, pink, or red. (Atlas of Vulvar Disorders, edema).Skin texture with edema is often smoother than normal, stretched out and fluctuant.
Is the integrity of the skin normal?
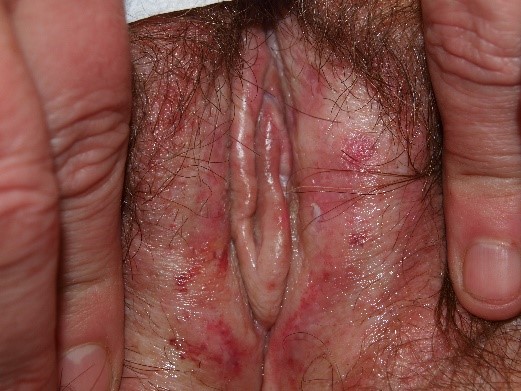
Normal, intact skin is not cracked, split, ulcerated, or otherwise disrupted. Many conditions cause scratching that results in excoriations, defined as fine erosions.
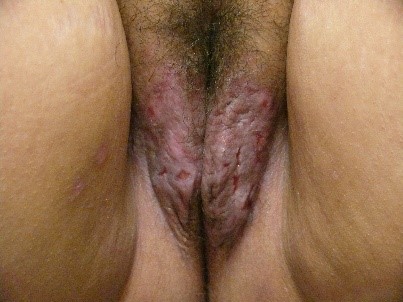
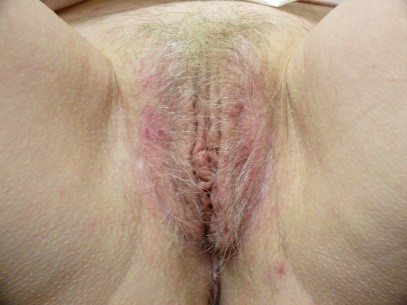
Fissures are also fine, linear erosions.
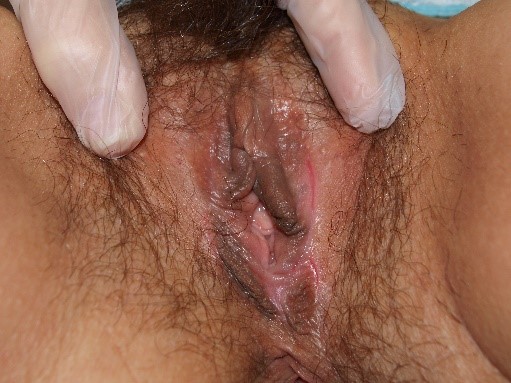
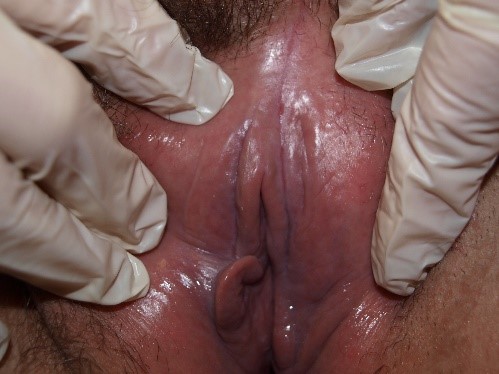
Erosions are shallow defects in the epidermis not extending to the dermis.
Blisters that have collapsed result in erosions, often with characteristic “collarettes” of raised, sometimes crusted tissue, surrounding them.

Ulcers are deeper defects, extending into the dermis and sometimes the subcutis, often affecting connective tissue and blood vessels, causing dark red, adherent material at the bases and edges.
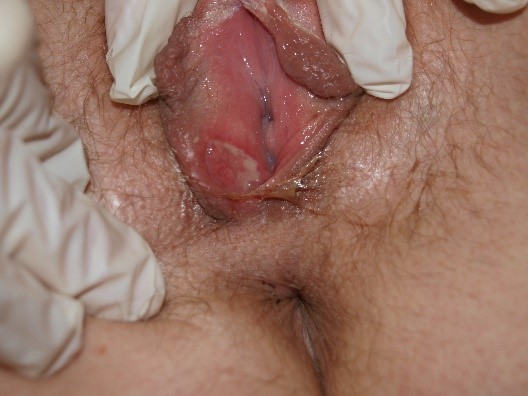
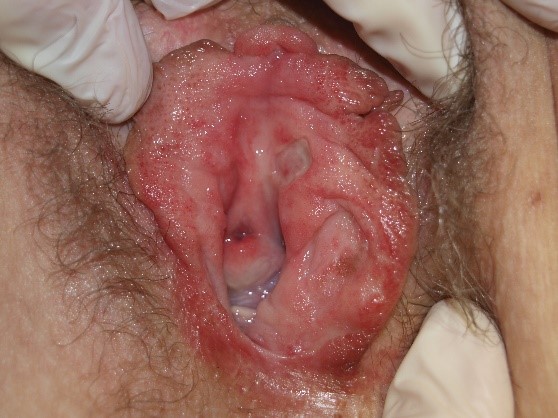
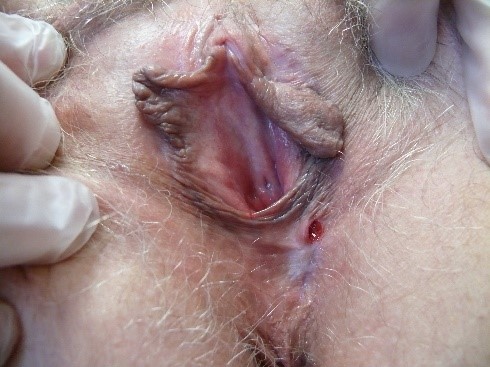
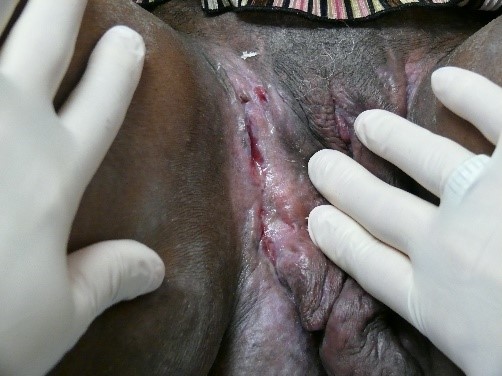
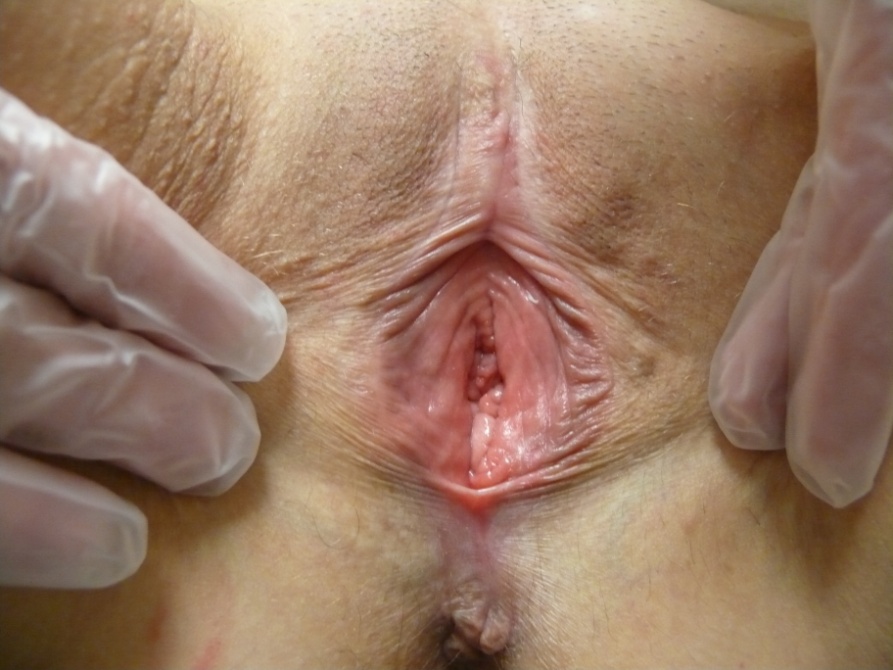
The final visual sweep
The final visual sweep during the vulvar exam involves consideration of how many conditions are present at the same time or whether the same condition looks different in different areas. Classical patterns of diseases found elsewhere on the skin may be absent in the vulvar area. For instance, in the case of psoriasis, scale may not look dry and silvery, but rather moist and white. The clinician who cultures regularly for yeast when encountering erythematous patches or plaques in the vulvar area and finds that the yeast cultures are negative, will do well to biopsy the skin for help with diagnosis. (These patches or plaques may be related to eczematous dermatitis, lichen simplex chronicus or dermatoses). Over time, with the aid of adjunctive testing (and review of texts on genital dermatology or the Atlas of Vulvar Disorders in this program), the clinician will begin to develop the eye necessary to recognize characteristic patterns based on morphology alone.
If a condition is present with alterations in more than one category (anatomical changes, color, texture, integrity), all of these must be kept in mind in moving forward to diagnosis. If the vulvar tissue looks completely normal but the patient experiences symptoms such as itching, burning, unprovoked pain, provoked pain, or dyspareunia, vulvodynia may be considered after repeat evaluations confirm normal skin, and associated tests and biopsy are negative. (Annotation K: Vulvar pain and provoked or unprovoked vulvodynia) Although Candida is certainly all too commonly blamed for women’s vulvovaginal problems, it must always be ruled out, whether seen under the microscope or not.
The final sweep also includes the use of the q-tip touch test for pain and other symptom mapping. This is covered in Annotation I and will not be covered here. It is very important, as well, to have the patient show you where she is symptomatic with her fingers, and not count solely on the q-tip touch test or on her attempt at verbal discription. We often ask the patient to show us where she is symptomatic before doing the q-tip test. The symptoms are often not where the patient described them as being!
The process of systematic data collection, just described, is called the “morphological approach” by experienced vulvovaginal specialists.41 42 In the “morphological approach,” one accumulates pure observations first, layering them with adjunctive facts and patient history to come to the diagnosis. Rather than searching for a skin condition by perusing lists of possible diagnoses, or even lists of possible groupings of etiologies, the practicing clinician defines what is seen, then pattern–matches with photographs or recollected similar lesions to come to a much smaller list of possible diagnoses.
An initial step toward doing this is learning and applying the vocabulary of the anatomical structures in describing what you see. Consistently naming the parts, e.g. labia majora, prepuce, clitoris, frenulum, interlabial sulci, labia minora, vestibule, etc, will help you to pinpoint the location of symptoms and signs. Precisely what body part is affected, what type of terrain? Is the anatomy normal or abnormal or are you not sure?
It is also essential to become intimate with basic descriptive dermatological terminology. As has been said, “Reading the skin is like reading a text. The basic skin lesions are like the letters of the alphabet: their shape, color, margination, and other features combined will lead to words and their localization and distribution to a sentence or paragraph.” 43 Others describe the process of morphological diagnosis as that of locating the “nouns,” (e.g. macules, papules, plaques, etc) and the “adjectives,” (red, skin-colored, brown, etc) and gradually building up the groups of likely diagnoses in that way.44
“Nouns” that describe texture and integrity of the skin
Lesion: a visible or palpable abnormality.
Rash: the word used colloquially to describe numerous or diffuse skin abnormalities. The lesions within a rash (exanthem) will resemble each other but may vary from being described as macular, papular, vesicular, urticarial, etc. The definitions below are more specific.
Macule: a small, flat area of color change, (< 1.0 cm in diameter). Not palpable. May be of any color.
Patch: an extension of a macule in length and width, (>1.0 cm); a non-palpable area of color change.
Papule: a small, elevated, palpable lesion (< 1.0 cm in diameter).
Plaque: an enlargement of a papule in length and width, (>1.0 cm). Elevated, palpable and flat-topped.
Lichenification: thickening of the skin with increased prominence of skin markings, usually from scratching or rubbing; excoriations may be seen. Scaling may or may not be present. Skin may be red, white, or skin-colored; the white color is a result of moisture retention in the thickened outer epidermis. This lesion may occur on normal skin or be part of an underlying skin disease.
Nodule: a large papule, (>1.0 cm); often hemispherical or poorly marginated; may be located on the surface, within or below the skin; may be cystic or solid.
Cyst: a closed cavity lined by epithelium that contains fluid or semisolid material.
Vesicle: a small, fluid-filled blister (< 0.5 cm in diameter) in which the fluid is clear to slightly cloudy. When the roof of a vesicle has been removed or has disintegrated, an underlying erosion remains visible.
Pustule: a vesicle that is packed with neutrophils and appears to be opaquely white. The fluid in a pustule (pus) is white or yellow.
Bulla: a large vesicle greater than 0.5 cm in diameter, containing clear fluid that is either contained in a single compartment or is multiloculated as a result of coalescence of multiple vesicles or bullae.
Crust: the irregular residue of exudates drying on the skin from disruption of the underlying epithelial barrier layer; may be thin or thick. The color will be yellow when made of dried serum, yellow or yellow-green when made of purulent material or brown, dark red or brown when made of old blood.
Scale: a hyperproliferative response of the epidermis; grey, white or silver in color looking like fine dust but presenting with palpable roughness.
Erosion: a shallow defect affecting the epidermis down to the basement membrane; the dermis is intact. Primary erosions: caused by trauma, usually scratching. These excoriations are linear or angular in shape, without “collarettes.” Secondary erosions: caused by breakdown or removal of a blister roof. These erosions are round and have a collarette of scale encircling the defect.
Fissure: a thin, linear erosion of the skin surface.
Ulcer: a deeper defect affecting the epidermis and some or all of the dermis.
Blisters or pustules (link to photos) may appear and then transition into surface disruption in the form of erosions or ulcers. (link to photos). The edges of these disruptions in the integrity of the skin may have either a raised or an undermined appearance that can contribute to diagnosis.
“Adjectives” that further describe what you see
Color: shades of pink, red, purple, white, brown, skin-colored, black, blue, yellow, orange
Number: single or multiple, individual or coalesced
Margination: sharply defined edges, diffuse and soft-edges
Configuration: symmetrical or irregular, round, amorphous, linear, serpinginous
Distribution: localized or generalized, unilateral or bilateral, keratinized or non-keratinized skin or both
Texture: thick, thin, smooth, crinkled, raised, bumpy, flat, flaking, crusty, soft, hard, degraded
Integrity: intact or disrupted
Number of conditions noted or number of variations of the same condition
The patient complains of itching and burning. You take her history. When you examine her, you look for anatomical abnormalities, you take note of the characteristics of any skin lesions, as above. You complete the vaginal exam and microscopy and then make a diagnosis. The following examples are focused on the history and exam of the skin. In each case, the history is the same to demonstrate that it is impossible to diagnose vulvovaginal conditions by the patient’s history alone.
Example 1: A 40 year old woman comes in with complaint of long-term vulvar itching and burning that she has assumed was yeast. She has been seen before and told she had yeast. She has tried over the counter creams and suppositories and was put on a course of weekly Diflucan, but these treatments have only helped temporarily. Her history is significant for sensitive skin and environmental allergies. You follow the steps of the algorithm, including her history, obtaining permission to do the exam, pain and symptom mapping, doing a vaginal pH, KOH, and wet prep. The pH is 4.0. Negative whiff.
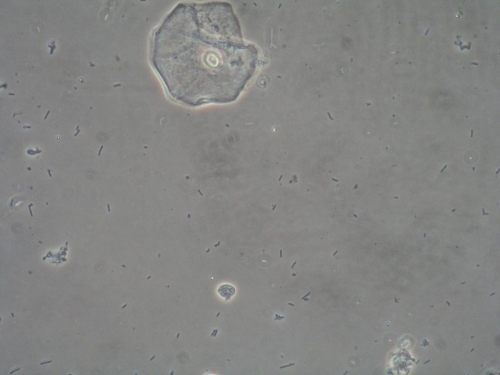
This is what you see under the microscope:
This is what you see on exam:
- Most prominent morphological characteristics: Architectural changes, White patches and plaques.
- On exam, you notice that the prepuce is fused to the glans clitoris. The labia minora are absent.
- There is no erythema. There are whitened strips of tissue on both sides of the clitoris and in the perineal area, where the texture is also crinkled like cigarette paper. The whitening extends down around the anus.
- The pH, KOH, and microscopy are normal.
The diagnosis is lichen sclerosus.
Differential: Yeast infection versus lichen sclerosus: All itching is not yeast. A major differential clue to lichen sclerosus is a) loss or alteration of normal anatomy and b) whitening of the skin. There may be other color changes to the skin with LS, as well: e.g. purple macules (purpura), erythema, and lichenification and there may be signs of post-inflammatory hyperpigmentation (patches of darkened skin). Candida never causes fusion of the prepuce to the glans clitoris or loss of the labia minora, which are both common with LS. Yeast could be present in any situation and should be cultured for, even if the wet prep and pH are normal. A biopsy would confirm this diagnosis, but in this case, the diagnosis is very clear.
Example 2: The same 40 year old woman with the same complaint of long-term itching and burning that she has thought was yeast, presents. She is symptomatic today. You follow the steps of the algorithm, including doing a vaginal pH, KOH, and wet prep. These tests are normal. When you do her exam, this is what you see:
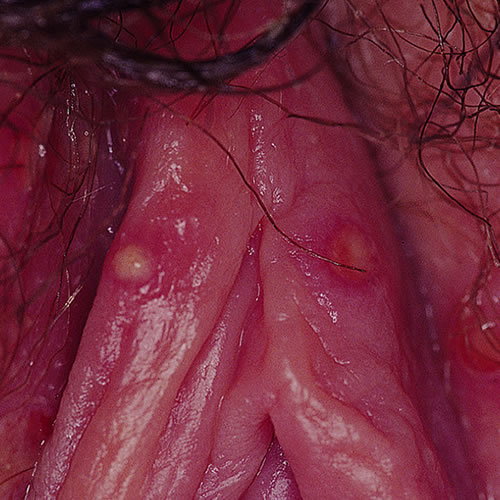
- Most prominent morphological characteristic: Blisters
- The anatomy is normal although erythema and edema are present.
- There is a full-blown blister on the patient’s right side and collapsing blisters on the left side.
The diagnosis is herpes simplex.
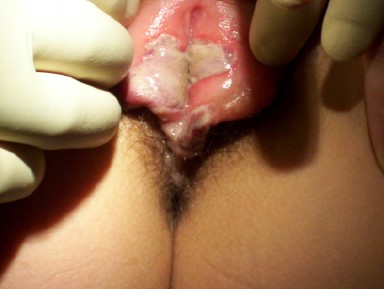
Differential: Herpes simplex versus yeast versus herpes zoster versus aphthosis: It is known that pain accompanies herpes outbreaks, but itching may also be a feature. Many women with an initial herpes infection assume that they have yeast, as do those with recurrent lesions. Erosions in the skin: either the typical collapsed blisters, or even tiny linear erosions that glisten, are typical of herpes simplex. The lesions are usually small, though they may coalesce. The blisters and ulcers of aphthosis are often large, covered with grayish exudate and are painful:
Herpes zoster follows dermatomes and is unilateral:
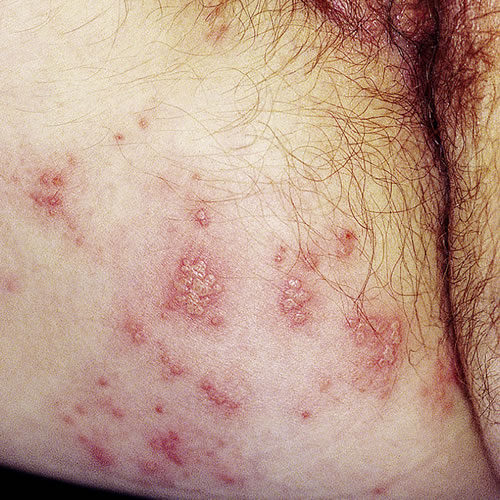
Herpes simplex may be unilateral or bilateral but it does not follow dermatomes. Again, yeast must always be ruled out in any situation.
Example 3: The same 40 year old woman presents with the same complaint of itching and burning, the same assumption about recurrent yeast. You follow the steps of the algorithm, including doing a vaginal pH, KOH, and wet prep. These tests are normal. When you do her exam, this is what you see:
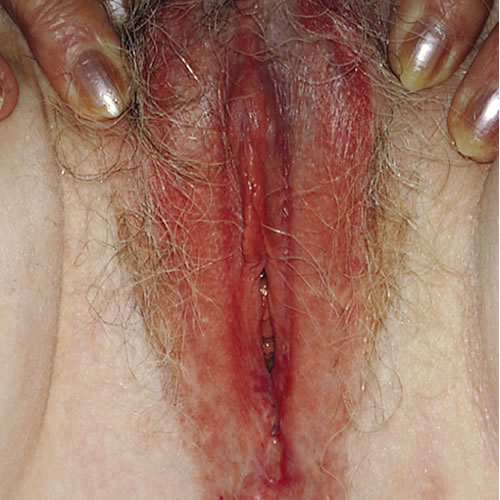
- Most prominent morphological characteristic: Red plaque.
- The anatomy is normal.
- The skin is reddened, with a sharply demarcated edge to the thickened erythematous area. There are no satellite pustules.
The diagnosis is chronic irritant contact dermatitis.
Differential: Yeast infection versus irritant contact dermatitis versus allergic contact dermatitis: Acute irritant dermatitis occurs as a reaction to a product or substance and causes a very quick reaction of erythema, swelling, sometimes blistering and erosions, usually producing irritation, burning and pain rather than itching. Chronic irritant contact dermatitis is a reaction to exposure to a substance over a more prolonged period of time with a slower build up of irritative symptoms. An allergic reaction is usually delayed 48 hours to days after exposure and results in itching, edema, and irritation, which can also produce vesicles, erosion, crusting, etc. Yeast is as described in Example 7. In some cases, biopsy must be done to distinguish the etiology and yeast culture must always be done if no yeast is seen under the microscope.
Example 4: The same 40 year old woman presents with long term itching and burning that she has thought was yeast. You follow the steps of the algorithm, including doing a vaginal pH, KOH, and wet prep. These tests are normal. When you do her exam, this is what you see:

- Most prominent morphological characteristics red plaque and lichenification.
- The anatomy is normal.
- The skin is thickened and reddened.
The diagnosis is lichen simplex chronicus.
Differential: Yeast infection versus lichen simplex chronicus: Lichen simplex chronicus is the end stage result of the itch-scratch-itch cycle that can originate in any of the vulvar skin problems that cause itching: yeast or other infection, irritant reactions, too-tight clothing, dermatoses, neoplasia, or even metabolic conditions. Repetitive scratching and rubbing cause thickening of the skin, increased skin creases, and linear excoriation marks, as well as broken hairs. Yeast may be present concomitantly.
Example 5: The same 40 year old woman presents with long term itching and burning that she has been told was yeast. You follow the steps of the algorithm, including doing a vaginal pH, KOH, and wet prep. These tests are normal. When you do her exam, this is what you see:
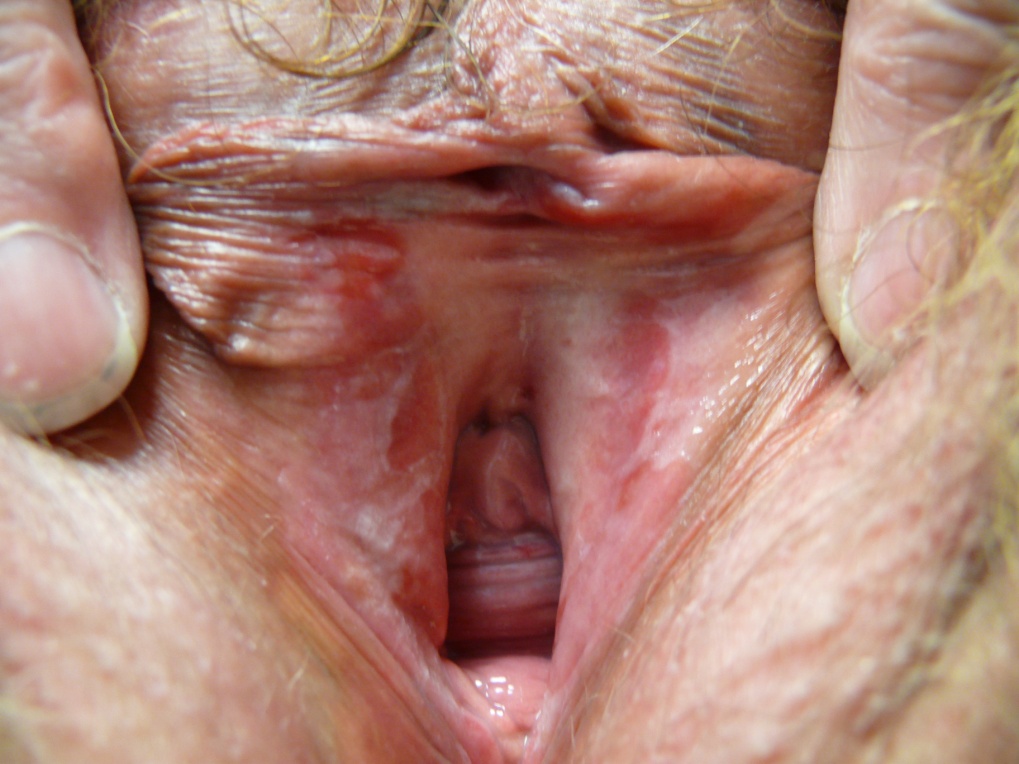
- Most prominent morphological characteristics: White plaque, erythematous erosions.
- The prepuce is moveable over the glans clitoris. The labia minora are flattened posteriorly though present anteriorly.
- There is both whitening of the skin and erythema. Some parts of the whitened skin are thickened and some have a lacy appearance. The whitening and erythema, which may represent shallow erosions, are most prominent in the vestibule.
The diagnosis is lichen planus.
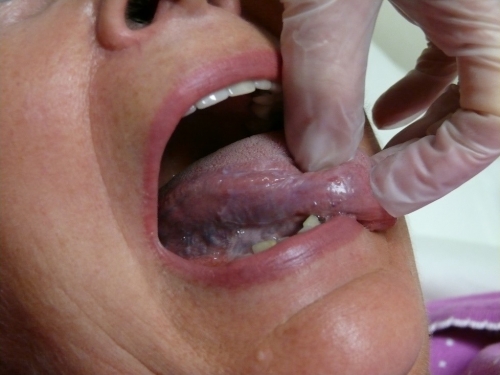
Differential: Lichen sclerosus versus lichen planus versus yeast infection: Lichen sclerosus almost always spares the vestibule, whereas the white, lacy reticulations of Wickham’s striae and bright red erosions in the vestibule are typical of lichen planus. In this case, an exam of the mouth and the rest of the skin, if not previously done, would be important since oral lesions, in particular, can make the diagnosis of lichen planus certain. Yeast culture, as always, should be done. Biopsy to confirm the diagnosis should be considered although a biopsy result that does not confirm LP does not negate the diagnosis.
Example 6: The same 40 year old woman with the same complaint: long-term itching and burning that she thought was yeast. You follow the steps of the algorithm, including doing a vaginal pH, KOH, and wet prep. These tests are normal. When you do her exam, this is what you see:
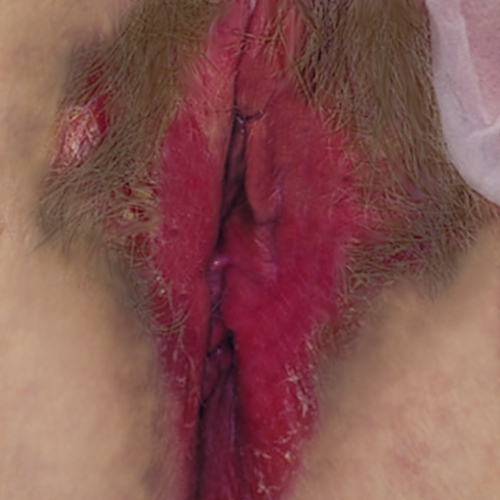
- The anatomy is normal.
- There is a plaque of reddened skin that is slightly raised, with a sharply demarcated edge. On close inspection, a fine scale is seen. The inner aspects of the labia minora are not affected.
- The patient points to the reddened area as being the area of her symptoms.
- You do a wet prep, KOH, and pH which are normal. No yeast is seen but you send a yeast culture. You may consider doing a biopsy.
The diagnosis is psoriasis.
Differential: Psoriasis versus tinea cruris versus yeast infection: One characteristic of psoriasis is a well-demarcated edge of the lesion, mostly affecting the hair-bearing vulvar skin in women and sparing the inner (mucosal) surfaces. It appears in thickened, red plaques, often with scale. It may also appear in another form (“inverse psoriasis”) in the areas of the skin folds. Tinea cruris also appears as reddened plaques with light scale, but is uncommon in women.

Yeast, in contrast, presents as thinner patches of erythema with more diffuse edges, often with tiny satellite papules and pustules scattered about the outer edges.
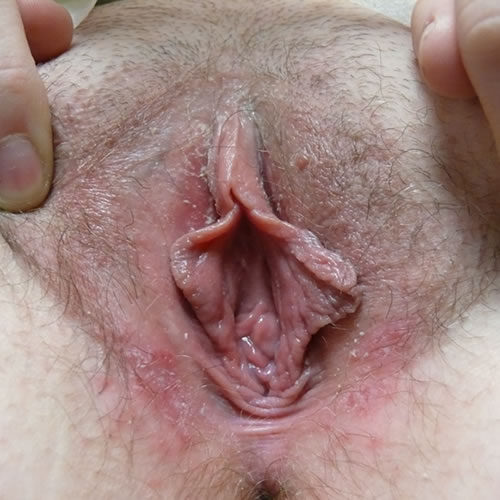
With yeast, there is usually erythema in the vestibule, as well.
Example 7: The same 40 year old woman presents with long term itching and burning that she has been told was yeast. You follow the steps of the algorithm, including doing a vaginal pH, KOH, and wet prep. These tests are normal. When you do her exam, this is what you see:
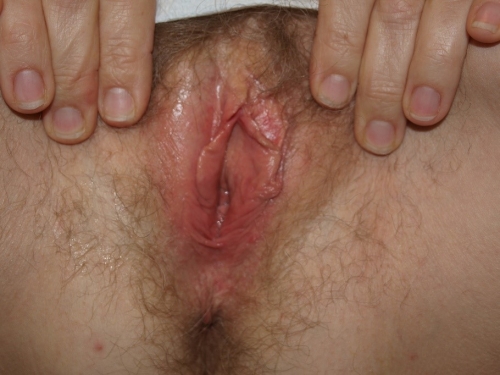
1. Most prominent morphological characteristics: Red patches, Satellite pustules.
2. The anatomy is normal.
3. The skin is erythematous without a sharply demarcated border. There may be small excoriations or fissures from scratching.
4. There are tiny satellite pustules which may or may not be present in the setting of Candidiasis.
The diagnosis is yeast infection (Candidiasis), probably due to C. Albicans
Differential: Irritant dermatitis, allergic or contact, yeast infection: The appearance of the skin with allergic or contact dermatitis is often more deeply reddened with varying degrees of surface changes: lichenification, fissuring, even erosions, depending on the duration of the reaction.
Yeast can still be present with a negative wet prep.
The Atlas of Vulvar Disorders, based on the work of Lynette J. Margesson, MD45 is set up in such a way that conditions are listed under their most prominent morphological characteristics, e.g. red papules and plaques, white patches, blisters, ulcers, etc. If a condition is likely to present in a number of different ways, it is shown under each category with an attempt made to find a photograph that demonstrates the variability in presentation. Rather than searching through lists of conditions to recognize the one at hand, the clinician undertakes pattern matching to locate the condition itself. Vulvovaginal diagnosis depends heavily on visual skills. It is therefore important to peruse the Atlas of Vulvar Disorders repeatedly to become familiar with the possible skin disorders that you will invariably see. Repetition will bring clarity.
The case studies will present you with opportunity for practice and include histories, physical exam findings, photographs, etc. that will allow you to experience using the Atlas of Vulvar Disorders on the practical level. (Case Studies). The diagnostic summary table worksheets will also be useful..
References
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.3-4.
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Clinical Dermatology, fourth edition. Malden, Massachusetts, 2008. 10.
- Iizuka H, Takahashi H, Ishida-Yamamoto A. Psoriatic architecture constructed by epidermal remodeling. J Dermatol Sci. 2004; 35:93-99.
- Kowalewski C, Kozlowska A, Gorska M et al. Alterations of basement membrane zone and cutaneous microvasculature in morphea and extragenital lechen sclerosus. Am J Dermatopathol . 2005; 27:489-496.
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.3-4.
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Clinical Dermatology, fourth edition. Malden, Massachusetts, 2008. 22.
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Clinical Dermatology, fourth edition. Malden, Massachusetts, 2008. 12.
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Clinical Dermatology, fourth edition. Malden, Massachusetts, 2008. 10.
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.11
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Clinical Dermatology, fourth edition. Malden, Massachusetts, 2008. 21.
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.11
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Clinical Dermatology, fourth edition. Malden, Massachusetts, 2008.21.
- Prussin C, Metcalfe DD (2003). IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 111 (2 Suppl): S486–494.
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.16
- Lynch P. Dermatology, 3rd edition. Baltimore: Williams and Wilkins; 1994.16
- Sargeant P, Moate R, Harris JE, Morrison GD. Ultrastructural study of the epithelium of the normal human vulva. J Submicrosc Cytol Pathol 1996;28:161–170.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006, p.43
- Foster DC, Vulvar disease. Obstet Gynecol. 2002; 100(1):145-163.
- Jones IS. A histological assessment of normal vulval skin. Clin Exp Dermatol. 1983;8:513– 521.
- Edwards L. Genital anatomy. In: Edwards L, Lynch P, eds. Genital Dermatology Atlas, Second Edition, Wolters Kluwer/Lippincott Williams & Wilkins. Philadelphia, 2011. 1.
- Woodruff JD, Friedrich EG Jr. The vestibule. Clin Obstet Gynecol. 1985;28:134–41.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 13.
- Nauth H. Anatomy and physiology of the vulva. In Elsner P, Marius J (eds): Vulvovaginitis. New York, Dekker, 1993,.1– 18.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 34.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 16.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 16.
- Johannesson U, Blomgren B, Hilliges M, Rylander E, and Bohm-Starke, N. The vulval vestibular mucosa—morphological effects of oral contraceptives and menstrual cycle. Br J Dermatol. 2007 Sep;157(3):487-493. Epub 2007 Jul 11.
- Kennedy CM, Turcea AM, Bradley CS. Prevalence of vulvar and vaginal symptoms during pregnancy and the puerperium. Int J Gynaecol Obstet. 2009 June;105(3):236-239.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 34.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 39
- Elsner P and Maibach HI. Microbiology of specialized skin: the vulva. Semin Dermatol. 1990 Dec;9(4):300-304.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 11.
- Edwards JN, Morris HB, Langerhans’ cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynaecol. 1985; 92:974.
- Turville S et al. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J Leukoc Biol. 2003; 74:710.
- Farage MA, Maibach HI, eds. The Vulva; Anatomy, Physiology and Pathology. Informa Healthcare, New York 2006. 17.
- McInnes IB. Role of cytokines in the immune system. In Up to Date. Rose B, ed. Up to Date, Waltham, MA. Dec 17, 2010.
- McInnes IB. Role of cytokines in the immune system. In Up to Date. Rose B, ed. Up to Date, Waltham, MA. Dec 17, 2010
- Kollias N. The physical basis of skin color and its evaluation. Clin Dermatol. 1995; 13:361.
- Lynch PJ. Genital pruritus and the eczematous diseases in Genital Dermatology Atlas, 2nd edition. Wolters Kluwer/Lippincott Williams & Wilkins. Philadelphia, 2011. 11.
- Lynch PJ, Moyal-Barracco, M, Scurry J, Stockdale C. 2011 ISSVD Terminology and Classification of Vulvar Dermatological Disorders: An approach to clinical diagnosis. Journal of Lower Genital Tract Disease, 16, 4, 2012. 339-344.
- Wolff K and Johnson RA. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology., sixth edition. New York, McGraw Hill, 2009, xxvii.
- ISSVD position paper, 2011.
- Fisher BK and Margesson LJ. Genital Skin Disorders; diagnosis and treatment. Mosby, St Louis. 1998