Introduction
Erythema multiforme (EM) is an acute, immune-mediated, self-limited, sometimes recurrent type IV hypersensitivity reaction characterized by the abrupt onset of “target”-shaped lesions on the skin.1 It presents with two forms of severity. Erythema multiforme minor is a localized eruption of the skin with minimal or no mucosal involvement. Erythema multiforme major (EM major) is, unlike Stevens- Johnson Syndrome (SJS) (SJS in the Atlas), rarely due to a drug exposure. It is most commonly precipitated by herpes simplex virus, Mycoplasma pneumoniae, or other infectious agents.2 3 Some believe that EM and SJS are the same disorder, but others feel that they are distinct conditions.4 5
Epidemiology
The incidence of EM is unknown, but as many as 1% of dermatologic outpatient visits are for EM. Globally, the frequency of EM is estimated at approximately 1.2-6 cases per million per year.6 It is slightly more common in males than females. It is rare in children younger than 3 years and in adults older than 50 years.7
Etiology
Medications are infrequent causes. Infectious agents are the major cause of EM. These include herpes simplex8 and Mycoplasma.9 Cases thought to be idiopathic are probably also due to bacterial or viral agents which have gone undetected.10 There have been reports of EM-like eruptions in patients with COVID-19.11
Symptoms and clinical features
In the mild form, EM minor, there is a spectrum of involvement, from few to no symptoms to a prodrome of weakness, fever, and malaise with sore throat. Target-like macules and papules, and occasional blisters, develop in crops over distal arms and legs in a symmetric fashion. There is little or no mucous membrane involvement. The lesions enlarge, forming secondary concentric rings giving the typical “target” or “iris” picture. There are vesicles but no bullae.
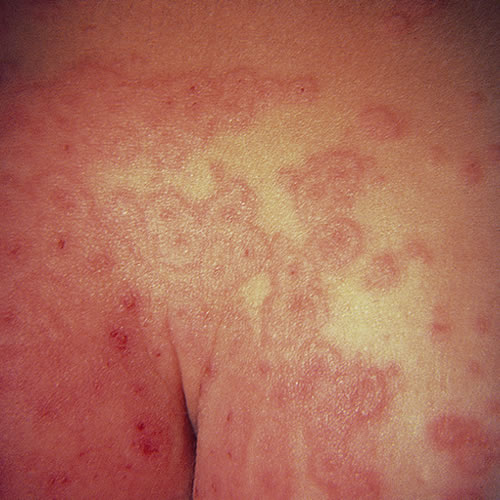
The lesions heal in 1 to 2 weeks, and the whole episode may last a month. These lesions can be itchy, burning, or painful.
With EM major, patients present with a history of fever, malaise, prostration, headache, cheilitis, photophobia, and sore throat that precede this blistering eruption by 2 to 3 days. Involvement of the buccal mucosa and lips is the most common, and lesions less commonly involve the ocular or genital mucosae.12 In the vulva, pain, burning, and dysuria are prominent complaints. Genital lesions include erosive and ulcerative vaginitis, vulvar bullae and vaginal synechiae. Long term sequelae such as labial agglutination and introital stenosis occurred in up to 28% of women in one series.13 Lesions are sharply defined flat papules with a central vesicle. Lesions may coalesce, resulting in erosions.
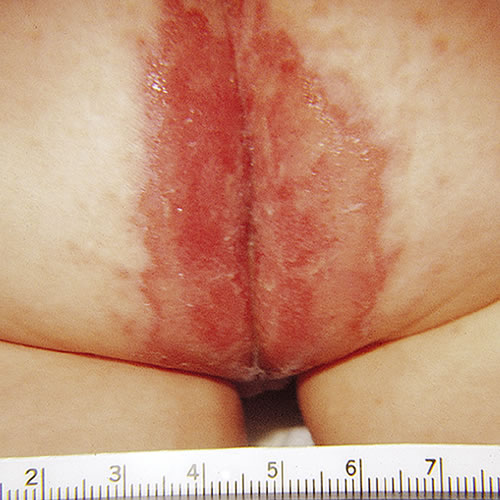
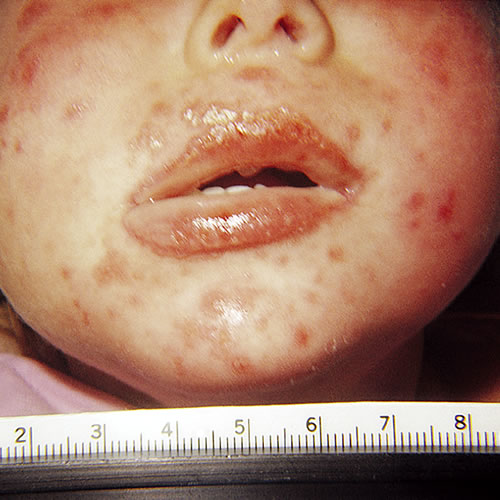
In some cases of blistering EM, lesions are found only on the palms, soles, and multiple mucous membranes, and prompt rupture of mucosal blisters results in erosions.
If widespread lesions exist, patients are toxic, risk secondary infection, and are similar in needs to burn patients.
EM may manifest with frequent recurrences, usually herpes related.
Diagnosis
A patient with vulvovaginal symptoms will have the history of target-like skin lesions, possibly ocular or oral lesions, and a history of an infectious agent known to produce this reaction. Recurrent EM minor is usually associated with an outbreak of herpes simplex preceding the EM outbreak by several days.
Pathology/laboratory findings
Inflammation is characterized by perivascular mononuclear infiltrates, edema of the upper dermis, apoptosis of keratinocytes with focal epidermal necrosis and sub epidermal bulla formation. In severe cases, complete necrosis of epidermis, as in toxic epidermal necrolysis, is seen.14 (Stevens-Johnson syndrome and Toxic Epidermal Necrolysis in the Atlas)
Differential diagnosis
The differential diagnosis includes drug eruption, psoriasis, secondary syphilis, urticaria, and generalized Sweet syndrome. Mucous membrane lesions may represent bullous diseases, fixed drug eruption, or primary herpetic gingivo-stomatitis.
Treatment
The major goal of treatment is to identify and treat any underlying cause. Mild cases of EM minor are self-limited and require no treatment. Patients with EM major should be managed with the help of a dermatologist. Vulvar or vaginal lesions are managed with warm soaks in plain water 3-4 times daily followed by a film of petrolatum. No other topical treatments should be used. Aggressive identification and treatment of secondary bacterial or Candidal infection and fluid loss are key.
There are no controlled trials of therapy for EM. Use of systemic steroids is controversial with strong advocacy by some,15 16 while others consider corticosteroids to be contraindicated.17 Early institution of corticosteroids can limit blistering, but sustained use or use after blistering has developed does not improve the condition and compromises the prognosis by increasing the risk of adverse reaction to the steroid.18
In severely ill patients, systemic glucocorticoids are usually given (prednisone 50-80 mg/day in divided doses, quickly tapered), but their effectiveness is not established.19
Class I (superpotent) topical steroids such as augmented betamethasone dipropionate 0.05% or clobetasol 0.05% have been used on the vulva and intravaginally in patients with EM, and on the vulva in patients with ulcerative lesions of SJS and TEN20 If the vulva is involved, start clobetasol 0.05% ointment applied in a thin film to the affected areas nightly. If the vagina is involved, prevention of strictures is important. Start the patient on hydrocortisone 10% in petrolatum 1 g per vagina nightly after use of a vaginal dilator (small Syracuse® or other Milex® dilator if there has been no sexual activity recently, medium for sexually active woman) for 20 minutes. These conditions can cause strictures very quickly and the dilator may be necessary in order to get the medication into the vagina. Steroids are continued until the acute inflammation resolves (~two weeks); the dilator should be used daily for two months or longer if necessary to keep the vagina patent. Dilator use in a virginal patient requires special consideration and individualization. If a young woman is comfortable with tampon use, a small dilator may be well tolerated. The alternative is use of the intravaginal steroid with an applicator every other day. One author recommends that no dilator treatment be initiated in the pediatric patient until she is an adolescent and able to manage the dilation process without emotional trauma.21 (dilator use in Annotation D: Patient tolerance of genital exam).
References
- French L and Prins, C. “Erythema Multiforme, Stevens-Johnson syndrome & Toxic Epidermal Necrolysis.” Dermatology, edited by Jean Bolognia, Elsevier, Limited. 2018.
- French L and Prins, C. “Erythema Multiforme, Stevens-Johnson syndrome & Toxic Epidermal Necrolysis.” Dermatology, edited by Jean Bolognia, Elsevier, Limited. 2018.
- Wetter DA. Erythema Multiforme: Pathogenesis, Clinical Manifestations, and Diagnosis. UpToDate, Wolter Kluwer, 2022.
- Assier H, Bastuji-Garin S, Revuz J, Roujeau JC. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. SOArch Dermatol. 1995;131(5):539.
- Giraud-Kerleroux L, Bellon N, Welfringer-Morin A, et al. Childhood epidermal necrolysis and erythema multiforme major: a multicentre French cohort study of 62 patients. C SOJ Eur Acad Dermatol Venereol. 2021;35(10):2051. Epub 2021 Jul 21.
- Plaza JA. Erythma Multiforme. In: James WD, ed. Medscape Reference Drugs, Diseases & Procedures 2011. http://emedicine.medscape,com/article/1122915
- Lam NS, Yang YH, Wang LC, Lin YT, Chiang BL. Clinical characteristics of childhood erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in Taiwanese children. J Microbiol Immunol Infect. Dec 2004;37 (6). 366-70.
- Huff JC. Erythema multiforme and latent herpes simplex infection. Semin Dermatol. Sep 1992; 11(3):207-210.
- Grosber M, Alexandre M, Poszepczynska-Guigne E, Revuz J, Roujeau JC. Recurrent erythema multiforme in association with recurrent Mycoplasma pneumoniae infections. J Am Acad Dermatol. 2007; 56(5 Suppl):S118-119.
- Wolff K, Johnson RA, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw Hill Medical, 2009. 148.
- Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, Suarez-Valle A, Saceda-Corralo D, Moreno-Garcia Del Real C, Fernandez-Nieto D SOClin Exp Dermatol. 2020;45(7):892. Epub 2020 Jun 25.
- French L and Prins, C. “Erythema Multiforme, Stevens-Johnson syndrome & Toxic Epidermal Necrolysis.” Dermatology, edited by Jean Bolognia, Elsevier, Limited. 2018.
- Niemeijer IC, van Praag MC, van Gemund N. Relevance and consequences of erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in gynecology. Arch Gynecol Obstet. 2009; 280:851-854.
- Wolff K, Johnson RA, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw Hill Medical, 2009. 148.
- Hynes A, Kafkala C, Daoud Y, et al. Controversy in the use of high dose systemic steroids in the acute care of patients with Stevens-Johnson syndrome. Int J Ophthamol Clin. 2005; 45(4):25-48.
- Yamane Y, Aihara M, Tatewaki S, et al. Analysis of treatments and deceased cases of severe adverse drug reactions-analysis of 46 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Jpn J Allergo. 2009; 58:537-547.
- Lissia M, Mulas P, Bulla A, Rubino C. Toxic epidermal necrolysis (Lyell’s disease). Burns. 2010 Mar;36(2):152-163.
- Edwards, L. Erosive and vesiculobullous diseases. In: Edwards L, Lynch PJ, eds. Genital Dermatology Atlas, 2nd ed. Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins, 2011. 149.
- Wolff K, Johnson RA, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw Hill Medical, 2009. 151.
- Amankwah YA, Haefner HK, Brincat CA. Management of vulvovaginal strictures/shortened vagina. Clin Obstet Gynecol. 2010; 53:125-133.
- Kaser DJ, Reichman DE, Laufer MR. Prevention of vulvovaginal sequelae in Stevens-Johnson syndrome and toxic epidermal necrolysis. Rev Obstet Gynecol. 2011; 4(2):81-85.