Introduction
Vulvar cancer comprises approximately 5% of all cancers in women, with 90% of those presenting as vulvar squamous cell carcinoma (VSCC).1
Development of primary VSCC takes place as a result of two separate pathways, one related to HPV infection, the other not related to HPV, but both forming precursor lesions that may lead to invasive disease.
Terminology and classification
Over the years, terminology has changed concomitant with knowledge. The following table outlines the changes in terminology over the years.
| Year | Terms |
|---|---|
| 1922 | The first description of vulvar epithelial neoplasia was reported as dyskeratose erythroplasiforme de la muquese.2 |
| 1943 | The terms vulvar carcinoma in situ and vulvar intraepithelial carcinoma were introduced. This led to the concept that every intraepithelial lesion carried a high neoplastic potential and should be removed by extensive surgery with adequate margins.3 |
| 1982 | The term vulvar intraepithelial neoplasia (VIN) was introduced.4 |
| 1986 & 2004 | The International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology Committee:5
VIN, usual type VIN (uVIN), HPV related and VIN, differentiated VIN (dVIN), HPV unrelated |
| 2014 | Lower Anogenital Squamous Terminology (LAST) classifications:6
LSIL (Low grade squamous intraepithelial lesion) and HSIL (High grade squamous intraepithelial lesion) and The concept of dVIN was left out |
| 2015 | The ISSVD Terminology Committee:7
Low-grade squamous intraepithelial lesion of the vulva (vulvar LSIL, flat condyloma, or HPV effect), HPV-related and self-limited and High-grade squamous intraepithelial lesion of the vulva (vulvar HSIL, VIN usual type), at risk for cancer development and Differentiated type VIN (dVIN), usually related to lichen sclerosus |
| 2020 | The World Health Organization Classification of Tumors Terminology: 8
HPV-associated (HPV+) squamous intraepithelial lesions: low grade squamous intraepithelial lesion of the vulvar (LSIL); high-grade squamous intraepithelial lesion of the vulva (HSIL) and HPV-independent (HPV-)VIN: differentiated vulvar intraepithelial neoplasia (dVIN); differentiated exophytic vulvar intraepithelial lesion (DEVIL); vulvar acanthosis with altered differentiation (VAAD) |
Epidemiology
Vulvar LSILs are related to infections with a low-risk HPV type (e.g. 6 or 11) and do not progress to invasive cancer. The prevalence has been reported as 107-229 per 100, 000 women.9 10
Vulvar HSIL (uVIN) is the most commonly diagnosed type of VIN (approximately 90%) and has increased in incidence over the last 2 decades, contributing to a higher incidence of vulvar cancer seen in higher income countries, mostly in women younger than age 60.11
Vulvar HSIL (uVIN) is found primarily in premenopausal women, with an average age at diagnosis of 46. A 2021 study showed that HPV vaccine given to those younger than 17 years can significantly reduce the risk of developing vulvar and vaginal cancer.12
Analysis of data from the Dutch Pathology Registry revealed that the incidence of HSIL (uVIN) was 2.39 per 100,000 woman-years (European standardized incidence rate (ESR)) between 1991-1995, and rose to 3.26 between 2006-2011, while the ESR for dVIN increased only from 0.02 to 0.08. The 10 year cumulative risk for VSCC was 10.3%: 9.7% for HSIL and 50.0% for dVIN.13
Differentiated type VIN (dVIN) accounts for 2-10% of all VIN diagnoses and is seen mostly in postmenopausal women, usually associated with vulvar dermatoses such as lichen sclerosus and, less frequently, lichen planus. Despite being diagnosed less frequently, dVIN is recognized in 36-79% of women who subsequently develop VSCC.14
Etiology and risk factors
VSCC develops through two separate and distinct etiologic pathways via VIN precursor lesions. One pathway is driven by human papillomavirus (HPV) infection, the other by inflammatory vulvar skin conditions.
Vulvar LSIL, although strongly related to human papillomavirus (HPV), (usually low risk types 6 and 11), is not a VIN precursor. It represents condyloma effect or reactive atypia and is a benign lesion.15 16
Vulvar HSIL or usual type VIN (uVIN)
The HPV-associated precursor lesion is vulvar HSIL (uVIN). Infection with a high-risk HPV type (16 and/or 18) is found in 80% of all HSIL lesions. This pathway accounts for approximately 30% of invasive vulvar squamous cell carcinoma (warty, basaloid type).17 18 Usual type VIN (uVIN) occurs most commonly in young women who smoke or have a history of multiple sexual partners.19 Women with a history of cervical intraepithelial neoplasia are also at higher risk for HSIL of the vagina and vulva, as are those who are immunocompromised. 20 Immunodeficiency of HIV is also associated with uVIN.21 The risk of progression to invasive cancer is 3-9%. The time to progression to VSCC by the precursor has been estimated as 41-43 months.
Differentiated VIN (dVIN)
The other pathway to the development of invasive VSCC occurs independent of HPV. The precursor lesion is differentiated VIN (dVIN). This pathway accounts for approximately 80% of invasive vulvar cancers. These cancers develop in older women (>65 years of age). Differentiated VIN is not a frequent diagnosis as an isolated lesion. It is usually observed associated with lichen sclerosus.
Although accounting for only 5% of vulvar dysplasia diagnoses, dVIN has a higher rate of progression to squamous cell carcinoma, shorter interval to progression, and higher recurrence rate than HSIL.22 The progression to cancer has been estimated to be 33%. The time to progression to VSCC by precursor is 6-22 months.
The mechanism for the development of HPV independent VSCC is unknown. To date, no infectious agent has been identified in association with dVIN.23 A germline mutation in the p53 gene is thought to be associated with ~90% of dVIN cases. Mutations in p53 and PTEN are often seen in these cancers.24 More recently recognized are two new HPV-independent precursor lesions that are TP53 wildtype, termed DE-VIL (differentiated exophytic vulvar intraepithelial lesion) and VAAD (vulvar acanthosis with altered differentiation).25 These are hypothesized to represent a possible third pathway to development of VSCC.
Several reports show a clear relationship between dVIN and lichen sclerosus (LS) in adjacent skin of VSCC.26 27 28 On the other hand, HSIL associated with LS without coexisting vulvar carcinoma is more likely to be uVIN.29
The true overall risk of women with vulvar lichen sclerosus developing cancer is unknown. (The risk has been estimated as 3-5% (pre-steroid era studies) and <3% (post-steroid era).30 31 A recent review showed the following risks: the incidence of VSCC ranged from 1.16 (95% CI 0.03-6.44) to 13.67 (95% CI 5.50–28.17) per 1000 person-years for LS. The absolute risk of developing VSCC in patients ranged from 0.0% (95% CI 0.0–5.52) to 21.88% (95% CI 9.28–39.97) with LS and was 1.16% (95% CI 0.1–4.1) with lichen planus (LP).32
Age increases risk. Women greater than 70 years of age with dVIN have an estimated 5.9% risk of developing VSCC. Whereas those under 50 have a 1.8% risk.33 The risk of VSCC increases with the duration of LS. One study estimated the risk of developing cancer: 1% at two years and 37% after 25 years of disease.34
Symptoms and clinical features
There are no screening strategies for the prevention of vulvar cancer through early detection of vulvar HSIL (uVIN).38 HPV-related HSIL is often subtle and asymptomatic. Symptoms such as pruritus or pain are observed in about 60% of patients.39 Lack of symptoms in the remaining 40% makes accurate anogenital inspection during routine gynecologic examination important.
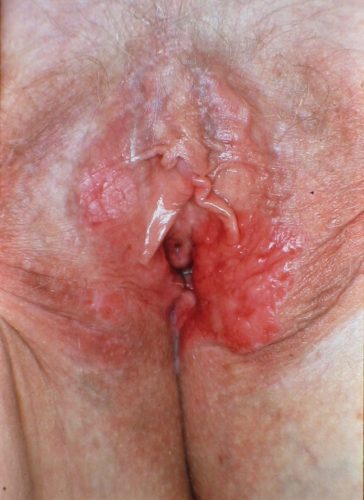
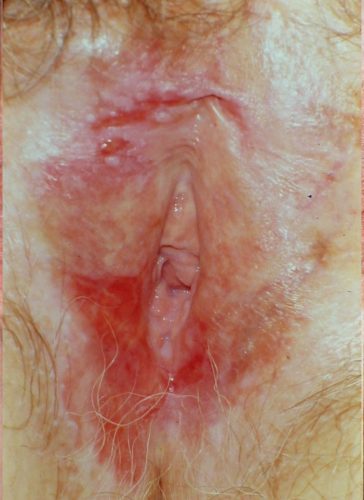
UVIN lesions are diverse in color and architecture and are often multifocal, ranging in size from 0.5 to 3 cm but sometimes coalescing to larger sizes. They often present as well-demarcated, skin colored, white, red, pink, brown, or black flat-topped papules and plaques.40 Any area of anogenital skin can be affected.
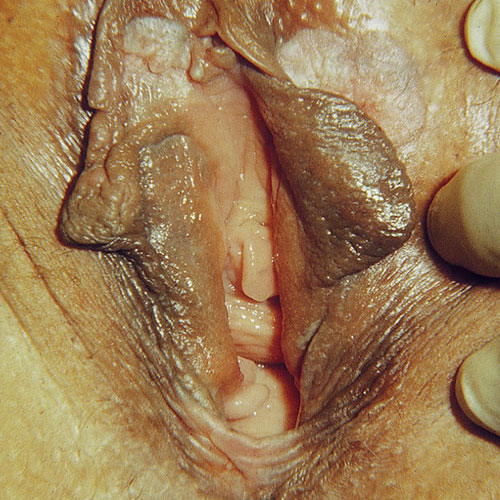
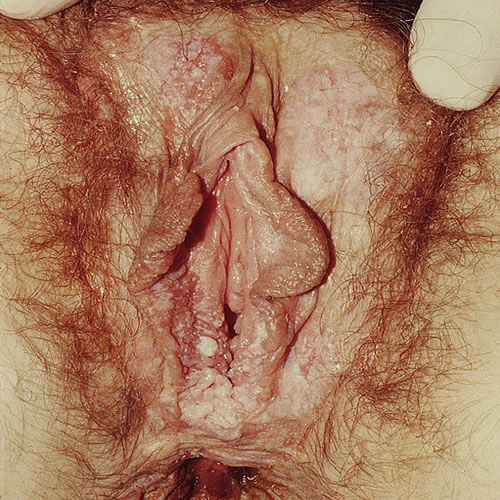
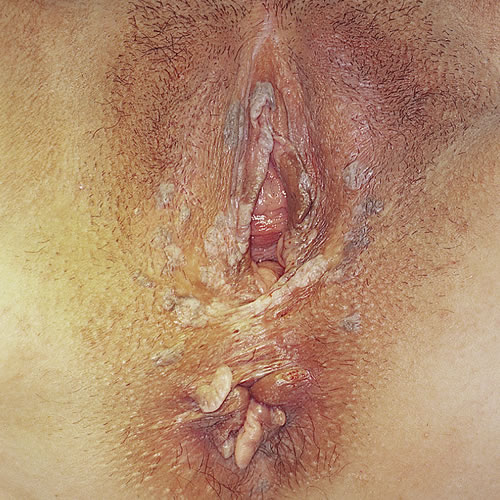
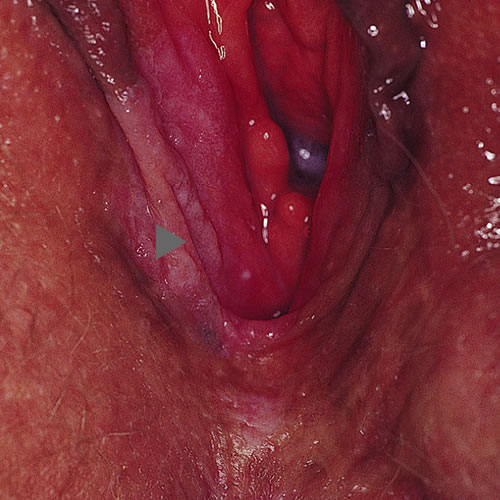
Patients with lichen sclerosus or lichen planus require life-time surveillance and treatment.41 There is no such thing as a cure for either disorder. DVIN is more likely to present with symptoms of itching, burning, or pain. It appears as a solitary red, brown, or pink sharply marginated plaque or nodule, from 2 to 5 cm in size, often with scale, crusting, or erosion, especially within the setting of lichen sclerosus or lichen planus in any anogenital area.42
Diagnosis
A careful examination includes the entire lower anogenital tract: the vulvar, perineal and perianal areas, as well as vaginal examination, cervical cytology and HPV screening, cervical colposcopy and biopsy if indicated. Digital anal/rectal exam is indicated in women over the age of 50.
Clinical suspicion can be aided by colposcopy or use of a hand-help magnifying glass and leads to biopsy. (Aceto-whitening has a high false positive rate and should be avoided by most clinicians). Biopsy of the most suspicious part of the lesion(s) should be performed under local anesthesia.43 [See Annotation G: Biopsy] Multiple biopsies are needed in multifocal disease to rule out invasion and not miss significant lesions. Red lesions and areas with hyperkeratosis, ulceration, or a rough and irregular surface are suspicious for dVIN44 which is often missed clinically and histologically.45 Patients are often symptomatic with a long history of lichen sclerosus or lichen planus and related vulvar itching and burning46 and the index of suspicion may be low for advancing disease.
Pathology/Laboratory Findings
uVIN
In HSIL, the epidermis is thickened and accompanied by hyperkeratosis and/or parakeratosis. There is loss of cell maturation with associated nuclear hyperchromasia, pleomorphism and mitotic figures at all levels.47 Underlying skin appendages may be involved.48
In warty HSIL, there are wide and deep rete ridges, similar to a condyloma. Cellular polymorphism and abnormal cell maturation are present. It is common to see acanthosis, abnormal mitotic figures, koilocytosis, multinucleation, and corps rounds.49
In basaloid HSIL there is thickened epithelium with a flat and non-papillomatous surface. In the epidermis there are large numbers of undifferentiated cells with a basaloid appearance. Numerous mitotic figures are present; koilocytic cells and corps rounds are not as frequent as in warty HSIL.50
DVIN
Because of the high degree of cellular differentiation and the lack of architectural disarray, dVIN can be confused with a benign dermatosis.51 Typically, the epithelium is thickened with parakeratosis, elongated and anastomizing rete ridges, and enlarged abnormal keratinocytes with premature eosinophilic cytoplasmic differentiation. Keratin pearl formation is commonly observed.52
Immunohistochemistry and genetic mutation analysis
Given the difficulties inherent in diagnosing VIN, several studies have recommended the use of p16 and p53 immunostaining for increased accuracy, with positivity for p16 supporting diagnosis of HSIL (uVIN) and a finding of p53 common in dVIN.53 54 55
Differential Diagnosis
Other vulvar malignancies (adenocarcinoma, melanoma, basal cell carcinoma, undifferentiated carcinomas, soft tissue sarcomas, metastatic cancers), benign neoplasms (condyloma, seborrheic keratoses), and inflammatory dermatoses (lichen sclerosus, lichen planus, lichen simplex chronicus).56 Paget disease is also a consideration.
Treatment/management
Two recent articles57 58 point out the importance of three steps in prevention of VSCC: 1) Primary prevention utilizing HPV vaccination, 2) Secondary prevention encouraging heightened awareness amongst clinicians for careful inspection of the anogenital tract, especially for those with possibly related signs and symptoms, a low index of suspicion for performing biopsy, and routine follow up of HPV positive cervical pathology with careful vulvar and vaginal exams. In addition, the authors recommend teaching self-examination to patients with chronic inflammatory vulvar skin conditions. 3) Their third preventive recommendation is treatment of high grade VIN lesions.
DVIN
Effective and compliant treatment of lichen sclerosus has been shown, in small studies, to reduce the risk of developing VSCC,59 the most common treatment being the ongoing use of topical corticosteroid ointments and strict surveillance. Excision with 0.5-1cm margins is the treatment of choice.60 61 And, because of frequent recurrence, these patients need to be followed by a specialist who has had training in managing vulvar disease.62
LSIL
LSIL does not require treatment as a neoplastic lesion. It is treated as condyloma. Failure of irritative changes to improve may necessitate reevaluation for another dermatologic abnormality or re-biopsy.
UVIN
Although spontaneous regression has been reported in 1.2% of women (usually <35 years old and pregnant women),63 uVIN should be considered a premalignant condition. Individualized treatment is recommended for all women with uVIN.
SURGERY AND CO2 LASER
Preserving normal anatomy and function of the vulva and preventing development of VSCC are essential treatment goals, and limited surgery, consisting of removal of all visible lesions, has become the accepted approach.64 65 Cold knife local excision with a 5 mm margin and 4 mm depth may be all that is needed in the case of focal lesions. UVIN, however is often multifocal, or may occur in the delicate clitoral, urethral, or anal areas, in which case CO2 laser vaporization to a depth of 1 mm is also an effective modality for biopsy-proven uVIN. While 1 mm depth is sufficient ablation for hair free epithelium, 3 mm depth is required in hairy areas of the vulva because the hair root sheet tends to extend as deep as 2.5 mm and is at significant risk for harboring HSIL.66 There is little difference in the risk of VIN recurrence between surgical excision and laser vaporization.67 The advantage of excision includes the presence of a histopathology specimen to rule out microinvasion.
MEDICAL THERAPY
Medical therapy has become a possibility for uVIN, provided that early invasive carcinoma has been ruled out by adequate biopsies. There is currently no evidence on how medical treatment compares with surgical treatment.68
Imiquimod: Imiquimod 5% cream produces a profound tumor-directed cellular immune response after binding on Toll-like receptors on the cell surface of dendritic cells, and stimulating secretion of pro-inflammatory cytokines.69
A Cochrane Database Review reveals that topical treatment with imiquimod may effectively treat about half of uVIN cases after a 16-week course of treatment, but the evidence on whether this effect is sustained is limited.70 In three randomized controlled trials, a complete response occurred in 36/62 (58%) in the imiquimod group. Moderate-quality evidence suggested that the complete response was sustained at one year and beyond, particularly in women with smaller VIN lesions.71
Imiquimod is applied to the individual lesions three times weekly for 16 weeks; it is used most commonly when there are clitoral lesions. Inflammation at the site of application can lead to erythema and erosion, often necessitating a reduction in application frequency to twice a week. An escalating dose regimen starting with an application once a week for two weeks, then twice a week for two weeks, then, if tolerated well, three times a week, is recommended.72
PHOTODYNAMIC THERAPY (PDT)
A tumor-localizing photo sensitizer, 5-aminnolevulinic acid, combined with non-thermal light, generates oxygen-induced cell death. PDT has been successful for non-melanoma skin cancer.73 It has the advantages of minimal tissue destruction, short healing time, and minimal side effects.74
Several nonrandomized and uncontrolled studies have shown response rates to PDT of 0-71%,75 76 77 with small unifocal lesions showing better response.78 A recurrence rate of 48% is comparable to that of surgery or laser therapy.79
Fifteen patients received PDT after injection of photosensitizer hematoporphyrin derivative Photogem and 630-nm red laser light for VIN, vaginal intraepithelial neoplasia (VAIN), or vulvar Paget’s disease between January 2003 and December 2013.The complete response rate was 80% (12/15) at the 3-month follow-up and 72.4% (10/14) at the 1-year follow-up.80
Combination treatment of 20 uVIN patients with sequential imiquimod combined with PDT showed an overall response rate of 55%. At one year, 65% were asymptomatic, compared with 5% at baseline.81
THERAPEUTIC VACCINES
Immunization with the quadrivalent or 9-valent human papillomavirus, which is effective against human papillomavirus genotypes 6,11,16 and 18, and 6,11,16,18,31,33,45,52, and 58 respectively, has been shown to decrease the risk of vulvar high grade squamous intraepithelial lesions (HSIL) (VIN usual type) and should be recommended for girls aged 11-12 years with catch-up through age 26 years if not vaccinated in the target age.82 Specifics can be found on the CDC website: https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html
Commercially-available vaccines are recommended to prevent HPV in healthy women, but do not elicit the cellular immune response necessary for HSIL treatment.83
OTHER TOPICAL TREATMENTS
5-fluorouracil, interferons, and indole-3-carbinaol have been used to treat uVIN with limited effectiveness or severe side effects that have eliminated them as standard therapy.84 85 86
One study showed equal efficacy in each of the major treatment methods (excision, CO2 laser ablation, or Imiquimod) but worse recurrence rates in those treated with a combination of excision and laser.87 Recurrence rates were also worse for smokers, those with larger lesion size or positive margins.
Follow up is long term and frequent for both uVIN and dVIN patients, with close surveillance of the entire anogenital area and a low threshold for performing colposcopy and biopsy.
References
- Griesinger LM, Walline H, Wang GY, Lorenzatti Hiles G, Welch KC, Haefner HK, Lieberman RW, Skala SL. Expanding the Morphologic, Immunohistochemical, and HPV Genotypic Features of High-grade Squamous Intraepithelial Lesions of the Vulva With Morphology Mimicking Differentiated Vulvar Intraepithelial Neoplasia and/or Lichen Sclerosus. Int J Gynecol Pathol. 2021 May 1;40(3):205-213. doi: 10.1097/PGP.0000000000000708. PMID: 32925443; PMCID: PMC7960553.
- Hudelo ML, Oury C. Dyskeratose erythroplasiforme de la muqueuse vulvaire. Bull Soc Franc Dermatol Et Syph 1922; 29:139–42.
- Barclay DL, Collins CG. Intraepithelial cancer of the vulva. Am J Obstet Gynecol 1963;86:95–106.
- Crum CP, Fu YS, Levine RU, Richart RM, Townsend DE, Fenoglio CM. Intraepithelial squamous lesions of the vulva: biologic and histologic criteria for the distinction of condylomas from vulvar intraepithelial neoplasia. Am J Obstet Gynecol 1982;144:77–83.
- Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med 2005;50:807–10.
- Darragh TM, Colgan TJ, Thomas Cox J, Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, Wilkinson EJ, Zaino RJ, Wilbur DC; Members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013 Jan;32(1):76-115. doi: 10.1097/PGP.0b013e31826916c7. Erratum in: Int J Gynecol Pathol. 2013 Jul;32(4):432. Erratum in: Int J Gynecol Pathol. 2013 Mar;32(2):241. PMID: 23202792.
- Bornstein J, Bogliatto F, Haefner HK, Stockdale CK, Preti M, Bohl TG, Reutter J; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. J Low Genit Tract Dis. 2016 Jan;20(1):11-4. doi: 10.1097/LGT.0000000000000169. PMID: 26704327.
- International Agency for Research on Cancer. World Health Organization Classification of Tumours—Female Genital Tumours. 5th edition. IARC: Lyon, 2020.
- Lukasiewicz E, Aractingi S, Flahault A. Incidence et prise en charge des condylomes acuminés externes en médecine générale. Ann Dermatol Venereol 2002;129:9916 http://www.ncbi.nlm.nih.gov/ pubmed/12442095.
- Monsonégo J, Breugelmans J-G, Bouée S. Incidence, prise en charge et coût des condylomes acuminés anogénitaux chez les femmes consultant leur gynécologue en France. Gynécologie Obs Fertil 2007;35:107–13.
- Kang YJ, Smith M, Barlow E, Coffey K, Hacker N, Canfell K. Vulvar cancer in high-income countries: Increasing burden of disease. Int J Cancer. 2017 Dec 1;141(11):2174-2186. doi: 10.1002/ijc.30900. Epub 2017 Aug 30. PMID: 28730615.
- Dehlendorff C, Baandrup L, Kjaer SK. Real-World Effectiveness of Human Papillomavirus Vaccination Against Vulvovaginal High-Grade Precancerous Lesions and Cancers. J Natl Cancer Inst. 2021 Jul 1;113(7):869-874. doi: 10.1093/jnci/djaa209. PMID: 33377930; PMCID: PMC8246827.
- Thuijs NB, van Beurden M, Bruggink AH, Steenbergen RDM, Berkhof J, Bleeker MCG. Vulvar intraepithelial neoplasia: Incidence and long-term risk of vulvar squamous cell carcinoma. Int J Cancer. 2021 Jan 1;148(1):90-98. doi: 10.1002/ijc.33198. Epub 2020 Jul 22. PMID: 32638382; PMCID: PMC7689827
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clinics 2017; 44:3: 339-52.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clinics 2017; 44:3: 339-52.
- Bornstein J, Bogliatto F, Haefner H, Stockdale C, Preti M, Bohl T, Reutter J. The 2015 International society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. Obstet Gynecol 2016; 127:2:264-68.
- Thuijs NB, van Beurden M, Bruggink AH, Steenbergen RDM, Berkhof J, Bleeker MCG. Vulvar intraepithelial neoplasia: Incidence and long-term risk of vulvar squamous cell carcinoma. Int J Cancer. 2021 Jan 1;148(1):90-98. doi: 10.1002/ijc.33198. Epub 2020 Jul 22. PMID: 32638382; PMCID: PMC7689827.
- Xing D, Fadare O. Molecular events in the pathogenesis of vulvar squamous cell carcinoma. Semin Diagn Pathol. 2021 Jan;38(1):50-61. doi: 10.1053/j.semdp.2020.09.010. Epub 2020 Sep 25. PMID: 33032902; PMCID: PMC7749059.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clinics 2017; 44:3: 339-52.
- Jamieson A, Tse SS, Brar H, Sadownik LA, Proctor L. A Systematic Review of Risk Factors for Development, Recurrence, and Progression of Vulvar Intraepithelial Neoplasia. J Low Genit Tract Dis. 2022 Apr 1;26(2):140-146. doi: 10.1097/LGT.0000000000000662. PMID: 35249976.
- Jamieson DJ, Paramsothy P, Cu-Uvin S, et al. Vulvar, vaginal, and perianal intraepithelial neoplasia in women with or at risk for human immunodeficiency virus. Obstet Gynecol. 2006; 107:1023
- Terlou A, Blok LJ, Helmerhorst TJ, van Beurden M. Premalignant epithelial disorders of the vulva: squamous vulvar intraepithelial neoplasia, vulvar Paget’s disease and melanoma in situ. Acta Obstet Gynecol Scand 2010 Jun; 89(6):741-748
- Saglam O, Samavoa E, Somasdkar S, et al. No viral association found in a set of differentiated vulvar intraepithelial neoplasia cases by human papillomavirus and pan-viral microarray testing. PLoS One 2015; 10(4): e012529.
- Griesinger LM, Walline H, Wang GY, Lorenzatti Hiles G, Welch KC, Haefner HK, Lieberman RW, Skala SL. Expanding the Morphologic, Immunohistochemical, and HPV Genotypic Features of High-grade Squamous Intraepithelial Lesions of the Vulva With Morphology Mimicking Differentiated Vulvar Intraepithelial Neoplasia and/or Lichen Sclerosus. Int J Gynecol Pathol. 2021 May 1;40(3):205-213. doi: 10.1097/PGP.0000000000000708. PMID: 32925443; PMCID: PMC7960553.
- Griesinger LM, Walline H, Wang GY, Lorenzatti Hiles G, Welch KC, Haefner HK, Lieberman RW, Skala SL. Expanding the Morphologic, Immunohistochemical, and HPV Genotypic Features of High-grade Squamous Intraepithelial Lesions of the Vulva With Morphology Mimicking Differentiated Vulvar Intraepithelial Neoplasia and/or Lichen Sclerosus. Int J Gynecol Pathol. 2021 May 1;40(3):205-213. doi: 10.1097/PGP.0000000000000708. PMID: 32925443; PMCID: PMC7960553.
- Medeiros F, Nascimento AF, Crum CP. Early vulvar squamous neoplasia: advances in classification, diagnosis, and differential diagnosis. Adv Anat Pathol. 2005; 12:20-26.
- Scurry J, et al. Comparison of histological features of vulvar lichen sclerosis with and without adjacent squamous cell carcinoma. Int J Gynecol Cancer. 1997; 7:192-199.
- Eva LJ, Ganesan R, Chan KK, Honest H, Leusley DM. Differentiated-type vulval intraepithelial neoplasia has a high-risk association with vulval squamous cell carcinoma. Int J Gynecol Cancer. 2009; 19:741-744.
- Micheletti L., Preti M., Radici G., et al: Vulvar lichen sclerosus and neoplastic transformation. J Low Genit Tract Dis 2016; 20: pp. 180-183.
- Jones RW, Sadler L, Grant S, Whineray J, Exeter M, Rowan D. Clinically identifying women with vulvar lichen sclerosus at increased risk of squamous cell carcinoma: a case-control study. J Reprod Med. 2004 Oct;49(10):808-11. PMID: 15568404.
- Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Maaike C.G. et al. Cancer Epidemiol Biomarkers Prev August 1 2016 (25) (8) 1224-1230
- Leis M, Singh A, Li C, Ahluwalia R, Fleming P, Lynde CW. Risk of Vulvar Squamous Cell Carcinoma in Lichen Sclerosus and Lichen Planus: A Systematic Review, Journal of Obstetrics and Gynaecology Canada, Volume 44, Issue 2, 2022, P 182-192, ISSN 1701-2163, https://doi.org/10.1016/j.jogc.2021.09.023.
- Thuijs NB, van Beurden M, Bruggink AH, Steenbergen RDM, Berkhof J, Bleeker MCG. Vulvar intraepithelial neoplasia: Incidence and long-term risk of vulvar squamous cell carcinoma. Int J Cancer. 2021 Jan 1;148(1):90-98. doi: 10.1002/ijc.33198. Epub 2020 Jul 22. PMID: 32638382; PMCID: PMC7689827.
- ScurryJ, K. Vanin K, Östör A. Comparison of histological features of vulvar lichen sclerosis with and without adjacent squamous cell carcinoma. International Journal of Gynecological Cancer. First published: 11 June 2003 https://doi.org/10.1046/j.1525-1438.1997.00045.x
- Lee A, Bradford J, Fischer G. Long-term Management of Adult Vulvar Lichen Sclerosus: A Prospective Cohort Study of 507 Women. JAMA Dermatol. 2015 Oct;151(10):1061-7. doi: 10.1001/jamadermatol.2015.0643. PMID: 26070005.
- Tessier-Cloutier B, Pors J, Thompson E, Ho J, Prentice L, McConechy M, Aguirre-Hernandez R, Miller R, Leung S, Proctor L, McAlpine JN, Huntsman DG, Gilks CB, Hoang LN. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome. Mod Pathol. 2021 Feb;34(2):508-518. doi: 10.1038/s41379-020-00651-3. Epub 2020 Aug 13. PMID: 32792599.
- Tessier-Cloutier B, Pors J, Thompson E, Ho J, Prentice L, McConechy M, Aguirre-Hernandez R, Miller R, Leung S, Proctor L, McAlpine JN, Huntsman DG, Gilks CB, Hoang LN. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome. Mod Pathol. 2021 Feb;34(2):508-518. doi: 10.1038/s41379-020-00651-3. Epub 2020 Aug 13. PMID: 32792599.
- Committee Opinion No 675: Management of Vulvar Intraepithelial Neoplasia. Obstet Gynecol. 2016; 128(4):e 178-82.
- Van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3,322 published patients. Gynecol Oncol. 2005; 97:645-651.
- Lynch, P. Skin colored lesions and can resemble condylomata. In: Edwards, L, Lynch PJ., eds. Genital Dermatology Atlas, 3rd ed., Philadelphia, Wolters Kluwer 2018, p 59.
- Singh N, Ghatage P. Etiology, clinical features, and diagnosis of vulvar lichen sclerosus: a scoping review. Obstet Gynecol Int 2020;2020:7480754. doi: 10.1155/2020/7480754.
- Lynch, P. Skin colored lesions and can resemble condylomata. In: Edwards, L, Lynch PJ., eds. Genital Dermatology Atlas, 3rd ed., Philadelphia, Wolters Kluwer 2018, p 59.
- van de Nieuwenhof HP, van der Avoort IA, de Hullu JA. Review of squamous premalignant vulvar lesions. Crit Rev Oncol Hematol. 2008; 68:131.
- Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol. 2000;24:429-441.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clinics 2017; 44:3: 339-52.
- Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am Surg Pathol. 2000;24:429-441.
- Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001; 20:16-30.
- Benedet JL, Wilson PS, Matisic J. Epidermal thickness and skin appendage involvement in vulvar intraepithelial neoplasia. J Reprod Med. 1991; 36:608-612.
- Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001; 20:16-30.
- Terlou A, Blok LJ, Helmerhorst TJ, van Beurden M. Premalignant epithelial disorders of the vulva: squamous vulvar intraepithelial neoplasia, vulvar Paget’s disease and melanoma in situ. Acta Obstet Gynecol Scand 2010 Jun; 89(6):741-748.
- Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001; 20:16-30.
- Saglam O, Samavoa E, Somasdkar S, et al. No viral association found in a set of differentiated vulvar intraepithelial neoplasia cases by human papillomavirus and pan-viral microarray testing. PLoS One 2015; 10(4): e0125292.
- Goyal A, Zhang G, Yang B. Differential expression patterns of GATA3 in usual and differentiated types of vulvar intraepithelial neoplasia: potential diagnostic implications. Mod Pathol. 2018 Jul;31(7):1131-1140. doi: 10.1038/s41379-018-0021-y. Epub 2018 Feb 13. PMID: 29434343.
- Tessier-Cloutier B, Pors J, Thompson E, Ho J, Prentice L, McConechy M, Aguirre-Hernandez R, Miller R, Leung S, Proctor L, McAlpine JN, Huntsman DG, Gilks CB, Hoang LN. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome. Mod Pathol. 2021 Feb;34(2):508-518. doi: 10.1038/s41379-020-00651-3. Epub 2020 Aug 13. PMID: 32792599.
- Griesinger LM, Walline H, Wang GY, Lorenzatti Hiles G, Welch KC, Haefner HK, Lieberman RW, Skala SL. Expanding the Morphologic, Immunohistochemical, and HPV Genotypic Features of High-grade Squamous Intraepithelial Lesions of the Vulva With Morphology Mimicking Differentiated Vulvar Intraepithelial Neoplasia and/or Lichen Sclerosus. Int J Gynecol Pathol. 2021 May 1;40(3):205-213. doi: 10.1097/PGP.0000000000000708. PMID: 32925443; PMCID: PMC7960553.
- Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol.2005; 106:1319-26
- , , . Cancer of the vulva: 2021 update. Int J Gynecol Obstet. 2021; 155(Suppl. 1): 7–18. https://doi.org/10.1002/ijgo.13881
- Geisler AN, Ganz JE. WNL we never looked: vulvar carcinoma incidence after screening cutoff. Int J Womens Dermatol. 2024 Jan 3;10(1):e127. doi: 10.1097/JW9.0000000000000127. PMID: 38179153; PMCID: PMC10763985.
- Bradford J, Fischer G. Long-term management of vulval lichen sclerosus in adult women. Aust N Z J Obstet Gynaecol. 2010;50:148–152.
- Allbritton JI. Vulvar Neoplasms, Benign and Malignant. Obstet Gynecol Clin North Am. 2017 Sep;44(3):339-352. doi: 10.1016/j.ogc.2017.04.002. PMID: 28778635.
- Terlou A, Blok LJ, Helmerhorst TJ, van Beurden M. Premalignant epithelial disorders of the vulva: squamous vulvar intraepithelial neoplasia, vulvar Paget’s disease and melanoma in situ. Acta Obstet Gynecol Scand 2010 Jun; 89(6):741-748.
- Jones RW, Scurry J, Neill S, MacLean AB. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008; 198:496 e1-3.
- Hoang LN, Park KJ, Soslow RA, Murali R. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016 Jun;48(4):291-302. doi: 10.1016/j.pathol.2016.02.015. Epub 2016 Apr 23. PMID: 27113549; PMCID: PMC5518939.
- , , . Cancer of the vulva: 2021 update. Int J Gynecol Obstet. 2021; 155(Suppl. 1): 7–18. https://doi.org/10.1002/ijgo.13881
- Wright VC, Davies E. Laser surgery for vulvar intraepithelial neoplasia: principles and results. Am J Obstet Gynecol. 1987; 156:374.
- Wright VC, Davies E. Laser surgery for vulvar intraepithelial neoplasia: principles and results. Am J Obstet Gynecol. 1987; 156:374.
- Lawrie T, Nordin A, Chakrabarti M, et al. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev. 2016 Jan 5;(1):CD011837. Doi: 10.
- Lawrie T, Nordin A, Chakrabarti M, et al. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev. 2016 Jan 5;(1):CD011837. Doi: 10.
- Iavazzo C, Pitsouni E. Athanasiou S, Falagas ME. Imiquimod for treatment of vulvar and vaginal intraepithelial neoplasia. Int J Gynecol Obstet. 2008; 101:3-10.
- Lawrie T, Nordin A, Chakrabarti M, et al. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev. 2016 Jan 5;(1):CD011837. Doi: 10.
- Lawrie T, Nordin A, Chakrabarti M, et al. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev.2016 Jan 5;(1):CD011837. Doi: 10.
- Le T, Menard C, Hicks-Boucher W, et al. Final results of a phase 2 study using continuous 5% Imiquimod cream application in the primary treatment of high-grade vulva intraepithelial neoplasia. Gynecol Oncol. 2007; 106:579.
- Martin-Hirsch P, Kitchener HC, Hampson IN. Photodynamic therapy of lower genital tract neoplasia. Gynecol Oncol. 2002; 84:187-189.
- Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, Stern PL, Moore JV, Corbitt G, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001; 61:192-196.
- Kurwa HA, Barlow RJ, Neill S. single episode photodynamic therapy and vulval intraepithelial neoplasia type III resistant to conventional therapy. Br J Dermatol. 2000; 143:1040-1042.
- Zawislak A, Donnelly RF, McCluggage WG, Price JH, McClelland HR, Woolfson AD, et al. Clinical and immunohistochemical assessment of vulval intraepithelial neoplasia following photodynamic therapy using a novel bioadhesive patch-type system loaded with 5-aminolevulinic acid. Photodiagnosis Photodynamic Ther. 2009; 6:28-40.
- Fehr MK, Hornung R, Degen A, Schwarz VA, Fink D, Haller U, et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Sur Med. 2002; 30:273-9.
- Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006; 100:271-275.
- Winters U, Daayana S. Lear JT, Tomlinson AE, Elkord E. Stern PL, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res. 2008; 14:5292-5299.
- Lawrie T, Nordin A, Chakrabarti M, et al. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst Rev.2016 Jan 5;(1):CD011837. Doi: 10
- Winters U, Daayana S. Lear JT, Tomlinson AE, Elkord E. Stern PL, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res. 2008; 14:5292-5299.
- Committee Opinion No 675: Management of Vulvar Intraepithelial Neoplasia. Obstet Gynecol. 2016; 128(4):e 178-82.
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J. Med. 2009; 361:1838-1847.
- Sillman FH, Sedlis A, Boyce JG. A review of lower genital intraepithelial neoplasia and the use of topical 5- fluorouracil. Obstet Gynecol Surv. 1985; 40:190-220.
- Spiros NM, Smith LH, Teng NN. Prospective randomized trial of topical alpha-interferon (alpha-interferon gels) for the treatment of vulvar intraepithelial neoplasia III. Gynecol Oncol. 1990; 37:34-38.
- Naik R, Nixon S. Lopes A, Godfrey K, Hatem MH, Monaghan, JM. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2006; 16:786-90.
- Wallbillich JJ, Rhodes HE, Milbourne AM, Munsell MF, Frumovitz M, Brown J, Trimble CL, Schmeler KM. Vulvar intraepithelial neoplasia (VIN 2/3): comparing clinical outcomes and evaluating risk factors for recurrence. Gynecol Oncol. 2012 Nov;127(2):312-5. doi: 10.1016/j.ygyno.2012.07.118. Epub 2012 Aug 4. PMID: 22867736; PMCID: PMC3632361.