Annotation G: Vulvar biopsy
Click here for Key Points to AnnotationBiopsies can be of great value in diagnosing a patient’s vulvar condition. Inspection of a tissue specimen by a trained dermatopathologist can confirm a clinical diagnosis already made, surprise you with a condition you had not thought of, or, while not being diagnostic, can give you clues as to how to treat. At the same time, many vulvar skin conditions have nonspecific histopathology, especially if taken in early or late disease. The biopsy result in these cases is not the whole answer; it is simply a piece of the puzzle to be taken into consideration in making the diagnosis, The biopsy is an interpretable test and needs to be considered together with clinical observations and history in deciding about both diagnosis and treatment. In some cases, (for instance removal of an entire lesion), the biopsy itself can also be the treatment.
A vulvar biopsy can be done painlessly with the right anesthetic and the right instrument, discussed below. Cervical biopsy forceps such as Tischler, Kevorkian, and Burke are inappropriate for vulvar use. They take large and deep samples, can avulse tissue and cause significant pain. Women who have had such biopsy experiences report them as traumatic and are often unwilling to submit to another vulvar biopsy, ever.
In our vulvovaginal specialty practice, we see patients who have usually seen multiple practitioners without resolution of their symptoms before coming to us. In the case of these patients, we try to biopsy at the first visit and we biopsy more than one site if necessary.
We biopsy:
- to confirm a clinical diagnosis
- to obtain a diagnosis when we are not sure what we are seeing
- to verify cancerous or pre-cancerous conditions
- to obtain more information if treatment has failed
- to identify new and different lesions
Discuss your rationale for doing the test with the patient and give her a consent form that clearly states the reason for the biopsy, the benefits, the risks, and side effects. In addition, the patient should have a handout she can take home with her telling her the following:
- What to expect: no pain during biopsy, mild stinging, and burning afterwards. Spotting of blood may be seen for an hour or two. Approximately a week is necessary for healing. Chemical cautery agents such as silver nitrate will leave a “clot” of brownish material at the biopsy site and may leave a brownish spot on the skin, which should disappear over time. Occasionally, a small scar is still visible long after healing.
- How to treat the area: use tepid sitz baths two or three times daily. Pat dry. May apply Lidocaine 2-5% to the area. No other topical treatment is necessary. Rinsing the vulva gently with a squeeze bottle of warm water after urination is suggested for the first 48 hours.
- Reasons to call the office: bleeding that does not stop, fever, redness and swelling around the site, pain not controlled with ibuprofen or acetaminophen. (Patient handout on after-biopsy care).
Ask the patient about allergies to materials used, including cleansing materials or topical or local anesthetics. Ask the patient about bleeding disorders or the use of medications that may make her at risk for bleeding, such as Warfarin or Aspirin. It is safe to do a biopsy in most cases, even when patients take these medications. Patients with bleeding disorders may want to consult with their hematologists first.
Punch biopsy is the most commonly used technique in vulvar evaluation. Punch biopsies are taken with a cylindrical blade (Keyes-type) ranging from 3mm to 5mm in diameter. A sharp instrument is essential. If you do biopsies and your punch biopsy tools are not sharp, order from a different vendor.
Most biopsy samples are placed into a vial of 10% buffered formalin; these vials are kept at room temperature in most offices. If there is a question about diagnosing any of the immunobullous diseases, the biopsy sample must be split in two (or a second biopsy must be done) and one sample placed in a vial with Michel’s transport medium. The sample will be tested with immunofluorescence. Michel’s medium is kept in the lab, refrigerated, and must be requested prior to doing the biopsy. Once the biopsy is done, it may be held in Michel’s medium for up to five days before processing. The three main disease categories identified by direct and indirect immunofluorescence are vesiculobullous disorders, vasculitis, and autoimmune connective tissue disease. The test also identifies antibodies associated with pemphigus and pemphigoid.
Different types of lesions sometimes require different biopsy techniques: Shave biopsies are rarely carried out on the vulvar skin due to the fragile nature of the area. If done on thickened plaque, a #15 scalpel blade or razor blade is used to shave the lesion down to the dermis. Aluminum chloride, ferric chloride, or ferric subsulfate is applied to the base of the shave biopsy site as a chemical cautery agent, to produce less tissue damage than electrocautery or silver nitrate.1
Small lesions such as skin tags or lesions on a stalk can be removed with a curved iris scissor. Pigmented lesions where melanoma is a possibility need to be biopsied by elliptical excision with margins (see Melanoma in the Atlas of Vulvar Disorders for more information on doing biopsies when melanoma is expected), or punch biopsy. In mucosal and modified mucosal tissue, absorbable suture material such as Vicryl, may be used.
To remove a blister or papule intact, or to avoid crushing very thin skin, place a 5-0 suture from 3 o’clock to 9 o’clock under the lesion. Put tension on both sides of the suture material to elevate the lesion and excise under it.
- In the case of inflammation, try to select areas with “active” disease rather than areas that have had some healing.
- For blistering skin, choose new vesicles and blisters and avoid those with crusts, erosions, or ulcerations. Try to remove the whole blister or biopsy the edge, keeping the “roof” intact; include some normal skin attached.
- For ulcers, sample the epithelium 1-2 mm from the ulcer border, avoiding eroded tissue that will not provide useful information.
- For suspected malignancy, the biopsy needs to be wide and deep enough for good information. We use a 5 mm Keyes punch, rotating the punch to a depth of 4-5 mm for adequate evaluation. A single suture is often necessary to close a deep biopsy. More than one area may need biopsy.
- For some atypical lesions, the Keyes punch biopsy may not be adequate to obtain clear borders, necessitating a wider excision.
Patient safety
Prior to performing the procedure, a “time-out” for safety is prudent. The components of the time out include:
- Verification of patient identity
- Verification of the exact nature of procedure
- Verification of surgical site and side
- Review of allergies
- Verification of the presence of a signed informed consent
- Initiation of sponge and needle count, if indicated
Apply Eutectic Mixture of Local Anesthesia 2.5% Lidocaine and 2.5% Prilocaine (EMLA)2 or generic lidocaine 2.5% and prilocaine 2.5% in a thick layer (1-2.5 grams), and allow it to take effect (at least 10-15 minutes).
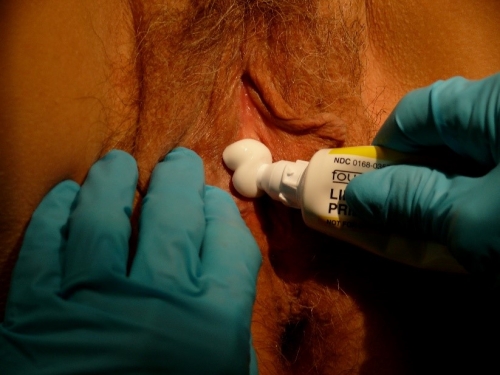
This topical cream can fully anesthetize some areas or partially anesthetize others, rendering the subsequently injected lidocaine much less painful. EMLA stings after application for up to 60 seconds. Local erythema and minor swelling may occur after application, but are temporary reactions. A rare patient may have an immediate irritant reaction with overwhelming burning, necessitating wiping the topical medication off.
On the non-keratinized epithelium of the vestibule or vagina, or thinly keratinized epithelium of the labia minora, prepuce, and in the perianal area, EMLA alone is often enough to anesthetize the area in 5-10 minutes. Keratinized skin of the labia majora, perineum, and buttock will take longer to anesthetize with EMLA (up to an hour) and usually requires injected Lidocaine.
By pre-arrangement, a woman can apply EMLA with occlusive plastic wrap on top to the designated site every half hour for two hours prior to a biopsy procedure. The pre-applied EMLA gives a sense of control, lessens anxiety, and minimizes pain with injection. Without pre-arrangement, she could do this in the office if both the patient and the clinician have the time.
Have available 1.0 cc of Lidocaine 1% along with 0.1cc of NaHCO3 (sodium bicarbonate, to buffer away the pain of the acidic xylocaine), drawn up with a 30 gauge needle. More lidocaine may be needed for multiple biopsies or for a highly anxious patient.
At the time of the biopsy, tell the patient that she will feel touching and movement as you wipe off the topical anesthetic.
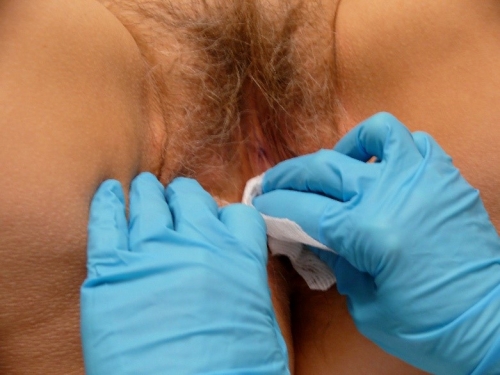
Indicate that you will test before the biopsy to make sure there is no pain. Ask, “Does this hurt?” while not touching at all. Then ask, “Does this hurt” while touching with a needle tip or with a small mouse-tooth pick up. Affirmative answer to the first question with negative to the second indicates anxiety but adequate anesthesia. Affirmative answers to both questions indicate anxiety and/or inadequate anesthesia and require injection as below.
Since a vulvar biopsy is done using clean, not sterile, technique, some clinicians do not perform skin preparation. If preferred, clean the skin with providone-iodine, isopropyl alcohol, or chlorhexidine gluconate.
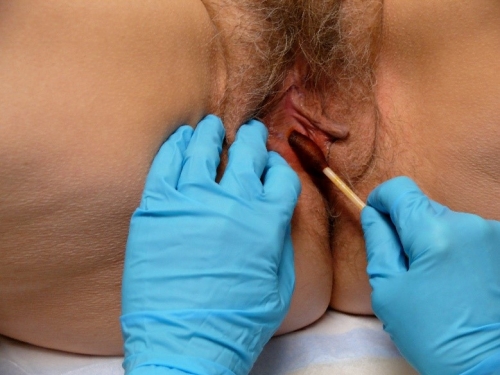
Make a wheal of a size that will cover the site that you intend to biopsy by injecting the Lidocaine/sodium bicarbonate mixture. You should not need to use the whole 1 cc of Lidocaine for a small sample.
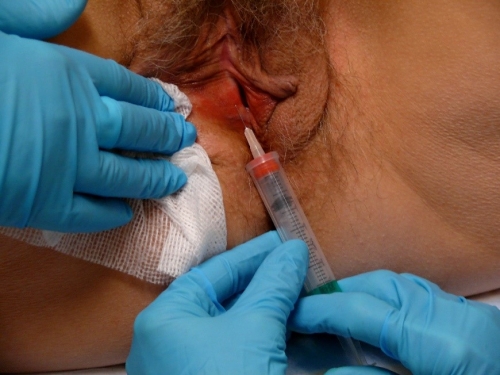
Locating the anesthetized site, apply the blade of the Keyes punch perpendicular to the tissue and use a downward pressure and rotary motion to cut down to a depth at least half way to the hub on the biopsy tool.
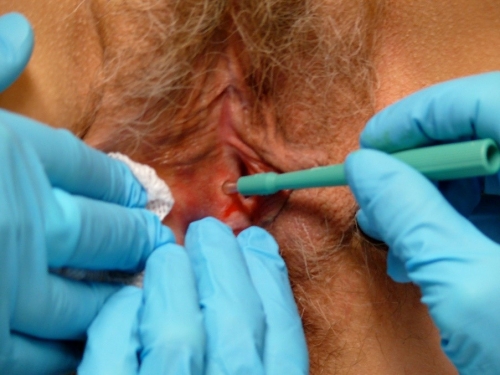
To remove the tissue from its base of attachment, stabilize the specimen with the needle or small pick-ups and clip off with iris scissors. Avoid crushing the tissue.
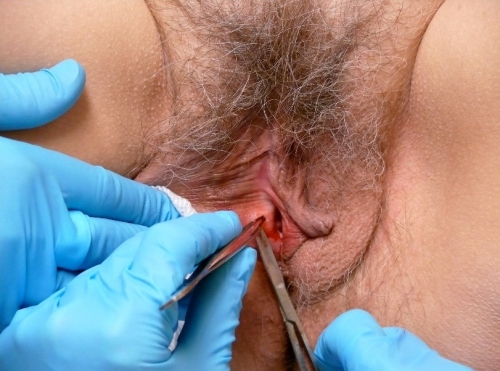
Place specimens in individual bottles of 10% buffered formalin solution, labeling and numbering them appropriately. Apply pressure to the site with clean gauze for a few minutes.
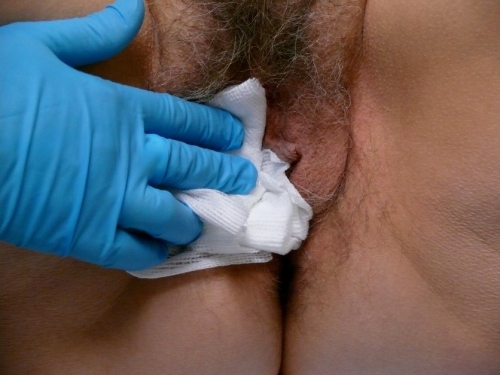
Silver nitrate or Monsel’s solution may be used to provide hemostasis although they may cause permanent discoloration of the skin with healing.
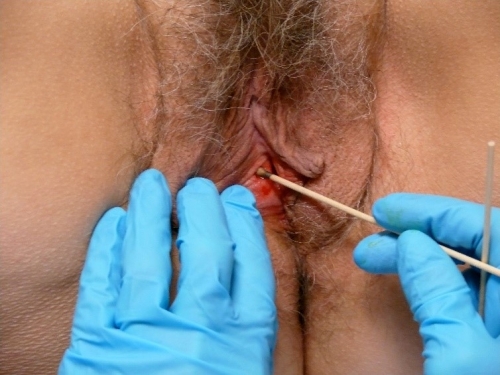
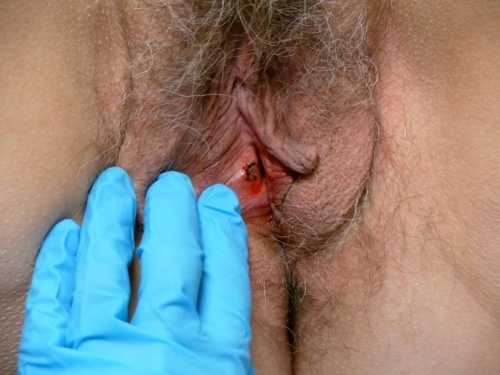
Occasionally, a stitch is needed to prevent excessive bleeding. In keratinized skin, Ethilon or Prolene may be used and will need to be removed.
When the site has clearly stopped bleeding, the patient is given information on the healing process and instructions for what to expect at home, what to do to minimize discomfort, how to call for questions or problems. (Patient handout on Biopsy.) An arrangement is made for communicating the results, usually by phone, and for follow up.
Inclusion of the history and physical findings on the pathology requisition will help the pathologist make a diagnosis. The most important clinical information includes location of the lesion(s), symptoms, arrangement of lesions (solitary, generalized, annular, linear, etc), clinical appearance (morphology), i.e. macule, papule, vesicle, plaque, etc), and color. Precise anatomical location of the sample can be helpful in predicting lymph node involvement in the case of a vulvar cancer.3 Midline or clitoral involvement is more likely in HPV-independent tumors.4
It is also helpful to state what diagnosis is suspected, such as granuloma for cutaneous Crohn disease, or squamous cell carcinoma in a field of lichen sclerosus. The specimen is received by the lab, chemically processed, and sliced into very thin fragments, stained and then inspected via light microscopy.
Occasionally, there can be bleeding after the patient leaves the office. Usually, pressure on the site with a piece of gauze (or just sitting on it) will stop the bleeding. If it continues, the patient may need to be seen again. Rarely, a stitch has to be placed to stop bleeding.
Infection is rare. Patients need to be warned to call for increased itching, pain, redness, drainage of pus, or swelling. If there is significant purulent material at the site of the biopsy, especially with cellulitis, the area can be cultured and antibiotics started.
It is helpful to be realistic about the amount of pain that will be present. Although the procedure is painless when done correctly, the patient can later expect pain similar to that caused by a cut sustained while cooking that does not need sutures. Acetaminophen, ibuprofen, or application of cool gel packs can be used for pain. The pain will be most pronounced for about two days and then start to improve. The wound, however, will take quite some time to heal completely and become skin-colored.
Dermatopathology is a subspecialty of dermatology and surgical pathology. Dermatopathologists understand the causes of epithelial disease at the cellular level and diagnose through the evaluation of skin biopsies. They are an invaluable adjunct to the clinical team involved in vulvovaginal care because they understand the complexity of the epithelium in general, the variety of epithelial surfaces present on the vulva (keratinized, non-keratinized, glabrous, and non-glabrous) and the possible pathology that can arise.
One expert dermatopathologist says, “The art of pathology is to be dogmatic about the diagnosis as often as possible, while not being afraid to hedge and give a differential diagnosis when the diagnosis is uncertain.”5 The diagnosis given by the dermatopathologist, being an interpretation of what he or she sees on the slide, may or may not give a clear answer to the patient’s condition, and conversation with the pathologist may be helpful to the clinician.
The clinician who evaluates and treats women with vulvovaginal complaints will find it helpful to understand terminology used to describe biopsy findings. The International Society for the Study of Vulvovaginal Disease (ISSVD) has clustered different descriptive terms into categories that represent the most common correlating disease conditions. (See Table G-1.) In dermatopathological reports, the terms at the left in the table are listed as being what the pathologist has seen to support his or her diagnosis. To non-pathologists, the vocabulary of the field of pathology is a foreign language. Experience with biopsy reports of women with vulvovaginal disease builds interest in understanding this language. Dermatopathology textbooks will give access to the histopathology photographs that elucidate the terminology (e.g., spongiosis, acanthosis), but consideration of the patient’s history, symptoms and signs, as well as the pathologist’s input (clinical correlation required), is essential for diagnosis.
Table G-1. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates (Table modified from the original)
| Name of finding | Definition | Common clinical correlates |
|---|---|---|
| Spongiosis | Intercellular edema between keratinocytes in the epidermis; keratinocytes may become elongated; often accompanied by exocytosis of lymphocytes and sometimes neutrophils or eosinophils. | Eczematous dermatitis (atopic, contact and allergic) |
| Acanthosis | Diffuse epidermal hyperplasia with increased thickness of the stratum spinosum. | Lichen simplex chronicus, Psoriasis, Reiter’s syndrome |
| Lichenoid pattern | A band-like infiltrate of inflammatory cells (usually lymphocytes) in the superficial dermis, parallel to the epidermis. | Lichen sclerosus, Lichen planus |
| Dermal sclerosis | Increased collagen with decreased numbers of fibroblasts causing thickening. | Lichen sclerosus |
| Vesiculobullous pattern | Immune deposits in skin may be bands of IgG or IgA at the basement membrane zone, of IgG deposits on the cell surface of keratinocytes. | Bullous pemphigoid, Cicatricial pemphigoid, Pemphigoid gestationis, Pemphigus vulgaris, Pemphigo vegetans |
| Acantholytic pattern | Loss of cohesion between keratinocytes because of dissolution of intercellular connections; may cause an intraepithelial vesicle. Keratinocytes are rounded rather than elongated and lymphocytes do not migrate into the epidermis. | Hailey-Hailey disease, Darier/s disease, Acantholytic dermatosis of the vulvocrural area |
| Granulomatous pattern | Granulomas are conglomerates of monocyte-derived histiocytes (macrophages); sometimes mixed with other inflammatory cells. Groups of these histiocytes may aggregate to form mulinucleated giant cells. Chronic granulomatous diseases are a genetically heterogeneous group of immunodeficiencies producing infectious and non-infectious disease in which phagocytic cells fail to kill organisms that they have engulfed because of defects in a system of enzymes that produce free radicals and other toxic small molecules. | Crohn disease, Melkersson-Rosenthal syndrome |
| Vasculopathic pattern | Non-inflammatory purpura: extravasation of RBCs into dermis. Narrowing or obliteration of small vessel lumina. Inflammation of blood vessel wall. | Aphthous ulcers, Behcet disease, Plasma cell vulvitis (vulvitis plasmacellularis) |
Adapted from: Edwards, L. guest editor, Vulvovaginal Dermatology; Dermatologic Clinics, Vol 28 (4), October 2010; chapter entitled, A histologic review of vulvar inflammatory dermatoses and intraepithelial neoplasm by Selim MA and Hoang MP. P 650 and from Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathological subsets and their clinical correlates. J Reprod Med. 2007; 52:3-9.6 7
- If patient is on steroids, inflammatory findings such as lichen sclerosus may not be confirmed. Two weeks off steroids prior to biopsy are essential.
- If you suspect malignancy, do the biopsy immediately. She may not come back for the procedure. Steroids do not mask malignancy. Do one or more biopsies depending on the number of suspicious areas. Push a 5 mm punch to the length of its hub for an adequate specimen. You may need a single suture to control bleeding.
- Make sure there is a specimen in the container before tightening the lid. Explaining “no specimen in container” to a patient is embarrassing.
- The patient needs to understand that biopsy does not always give a clear diagnosis and non-specific findings are possible.
- If the biopsy does not confirm what you suspect, the disease is not ruled out. Consider a treatment trial.
- Streamline your biopsy procedure: decision –EMLA on –go over consent – assistant brings in prefabricated biopsy kit – good to go.
References
- Edwards L, Diagnostic and therapeutic procedures, in Edwards L. and Lynch P. Genital Dermatology Atlas, Second Edition. 2011. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins: 27.
- http://www.drugs.com/pro/emla.html
- Hinten F, Molijn A, Eckhardt L, et al. Vulvar cancer: two pathways with different localization and prognosis. Gynecol Oncol 2018;149:310–17. 10.1016/j.ygyno.2018.03.003
- Hinten F, van den Einden LCG, Cissen M, et al. Clitoral involvement of squamous cell carcinoma of the vulva: localization with the worst prognosis. Eur J Surg Oncol 2015;41:592–8. 10.1016/j.ejso.2015.01.002
- Rapini RP. Practical Dermatopathology. Philadelphia: Elsevier,Mosby; 2005:1.
- Selim, MA and Hoang, MP. A histologic review of vulvar inflammatory dermatoses and intraepithelial neoplasm. In Edwards, L. guest editor, Vulvovaginal Dermatology; Dermatologic Clinics, Vol 28 (4), October 2010; 650.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathological subsets and their clinical correlates. J Reprod Med. 2007; 52:3-9.