Annotation P: Vaginal secretions, pH, microscopy and other testing
Click here for Key Points to AnnotationDespite the wide variety of etiologies for vulvovaginal symptoms, women and their providers continue to assume at the onset of itching, burning, dyspareunia and/or change in discharge, that “vaginitis,” usually Candida or bacterial vaginosis (BV), is to blame. Many women, under this assumption, inaccurately self-diagnose and self-treat.1 Many clinicians, under this assumption, still treat by telephone diagnosis, an unreliable practice.2 Patient self-testing for many conditions is being evaluated by researchers, but there is no standard of care that supports these methods as yet.3 4 5
Successful diagnosis and treatment of vulvovaginal disease is based on the importance of not making assumptions, of taking a careful history, and doing a careful examination, beginning with inspection of the vulvar skin for changes in architecture, color, texture, and integrity. (See Annotation H describing alterations of vulvar anatomy and epithelium (the morphologic approach) to identify etiologies of symptoms that may be outside the realm of “vaginitis.”) Next, precise symptom location and mapping with the Q-tip test are essential. The final information leading to correct diagnosis is obtained with pH and microscopic examination of the vaginal secretions. There are many efforts underway to create more accurate point of care tests that will reliably give a diagnosis on the spot,6 but pH, KOH, and microscopy give information that is outside the realm of commercial testing and the elimination of microscopy in clinical practice would be an injustice to patients and providers, alike.
In the past, clinicians were trained to diagnose by inspection only. However, the appearance of vaginal discharge is extremely unreliable and should never form the basis for diagnosis.7 Examination and diagnostic studies are necessary in all women. It is important to remember that conditions that affect the vulva may also manifest in the vagina and vice versa. In addition, multiple conditions may be present simultaneously. On the other hand, if the pH and wet mount microscopic evaluation are normal, many conditions can be automatically ruled out.
Complaints suggesting a vaginal etiology may include discharge, itching, irritation, pain, and dyspareunia in varying degrees. Start your history with your mind a clean slate, disregarding diagnoses in the patient’s chart. Ask about symptoms, not diagnoses, and obtain a time line of what happened first, what was done, whether it helped, and what happened next. (Anno B: The patient history) Patients frequently indicate that a treatment “didn’t work,” but close questioning may show that a steroid or anti-fungal provided significant relief at first, but the symptoms returned. Patients often do not allow adequate treatment time for a medication to “work.” Few know that fluconazole acts promptly against the Candida organism, but resolution of the inflammation takes far longer: days to weeks.
It is essential, in addition to the vaginal symptom time-line, to consider:
- A new sexual partner. A new sexual partner increases the risk of acquiring sexually transmitted diseases. In addition, with increased frequency of sexual relations with a new relationship, Candida albicans is common, as is bacterial vaginosis.
- Hygienic practices (e.g. daily use of panty liners, feminine products). Do a careful review of the woman’s personal practices: bath soap and method of washing, menstrual protection, use of any products in the vulvar area, clothing practices, daily physical activities (biking, horseback riding) that may affect the vulva. Exogenous agents may cause vulvar symptoms mistakenly attributed to an infectious source.
- Medications (prescription and nonprescription) used. Antibiotics and high-estrogen contraceptives may predispose to Candida vulvovaginitis. Increased physiologic discharge can occur with estrogen-progestin contraceptives leading a woman to suspect infection. Irritant reactions to topical products or medications may cause pruritus unresponsive to antifungal agents.
- Relation of symptoms to menses. Candida vulvovaginitis often occurs in the premenstrual period, while trichomoniasis often flares during or immediately after the menstrual period. Hormonal states related to absence of menses in lactating women cause vaginal atrophy as severe as in the postmenopausal period, though temporary.
- Presence of abdominal pain suggestive of pelvic inflammatory disease. Suprapubic pain is suggestive of cystitis. Both suprapubic and abdominal pain, uncommon with vaginitis, may represent symptoms requiring a separate work up, including ultrasound.
- Hormonal therapy in menopausal woman. Absence of adequate estrogen may cause uncomfortable vaginal atrophy. Systemic oral or topical hormonal therapy may still be inadequate for vaginal estrogen levels, leading to dyspareunia. In addition, use of estrogen in the postmenopausal woman can lead to Candida.
History alone does not allow a definitive diagnosis since there is considerable overlap among the different disorders.8 Examination and some diagnostic studies are necessary in all women.
Steps of the physical exam have preceded this annotation in Annotations C (The targeted, non-genital exam), E (Detailed vulvar exam), F (The vulvar architecture), H (The vulvar epithelium), and I (Pain and symptom mapping), as well as the Annotation M (Speculum exam and exam of the cervix), N (The vaginal architecture), and O (The vaginal epithelium). Vaginal discharge may be present at the introitus, may coat the tissue of the vestibule, or may only be assessed on speculum exam.
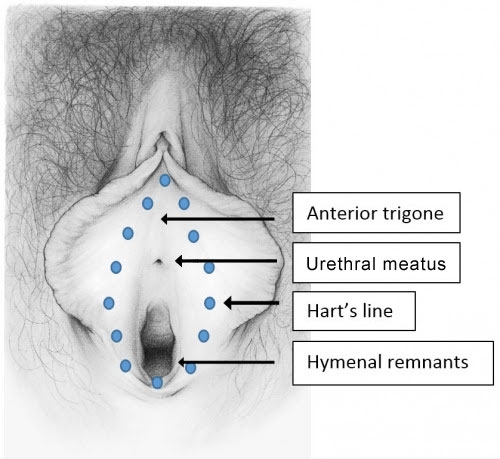
See the following photographs for a sense of visual inspection of the vestibule:
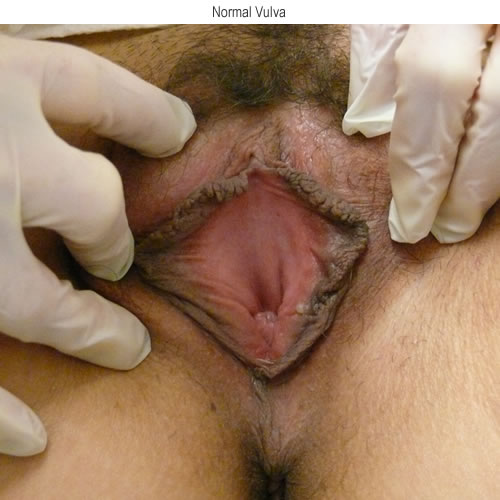
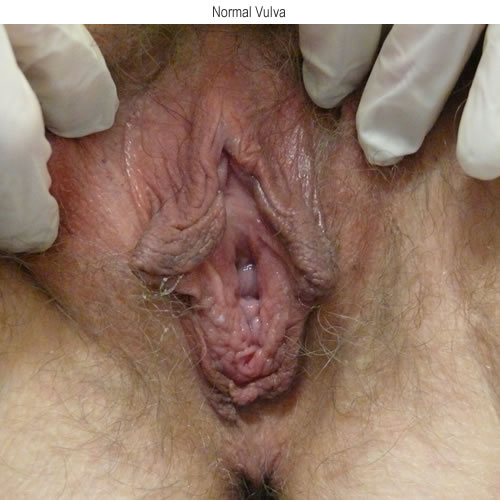
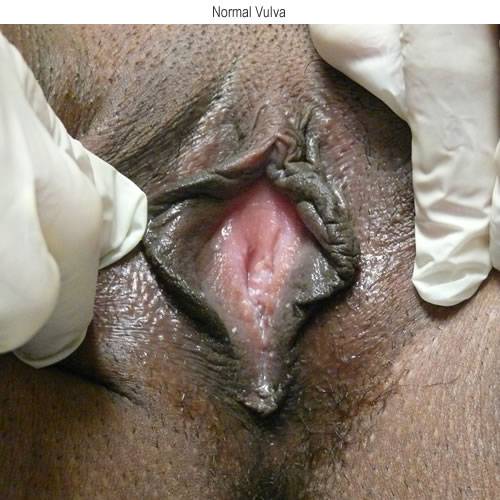
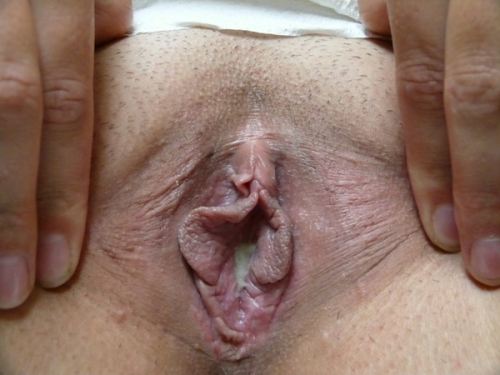
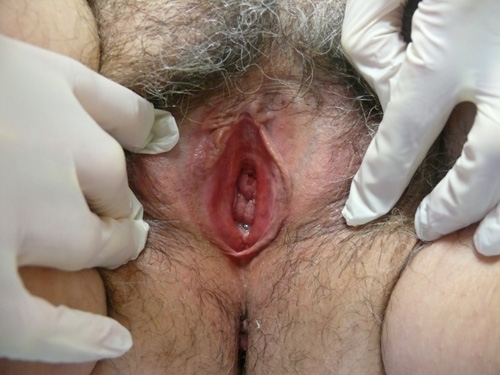
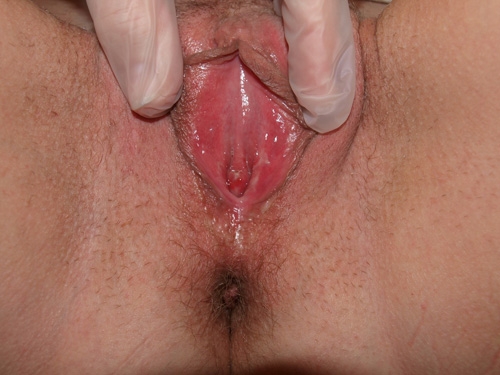
Diagnosis of a specific type of “vaginitis” should never be made based on the appearance of the discharge alone. Clinicians are classically taught that certain characteristics of discharge can be linked with particular conditions. For instance, we expect that discharge can be white and clumpy with yeast, greenish and purulent with cervicitis or pelvic inflammatory disease (PID), greenish-yellow and frothy with trichomonas, thin, homogeneous, gray-white and malodorous (fishy-smelling) with bacterial vaginosis, or tan or brownish, with the presence of old blood. However, the appearance of both normal and abnormal discharge can be highly variable and all of the conditions listed can be found in the absence of significant discharge.
Origin and composition of vaginal secretions
Secretions from the vagina in women of reproductive age are normal. They do not, however, originate from the vagina, which has no glands.
Fluid enters the vaginal cavity by transudation through the vaginal epithelium as 90–95% water. It contains secretions from Bartholin’s and Skene’s glands of the vulva, located close to the vaginal orifice, cervical mucus, endometrial and tubal fluids. In addition, the fluid may also contain some residual urine and exfoliated epithelial cells. As a consequence, organic and inorganic salts, urea, carbohydrates, mucins, fatty acids, albumin, immunoglobulins, and other macromolecules can be found in vaginal fluid. Fluid entering the vagina, as well as the vaginal resting fluid volume, are subject to inter- and intraindividual variability and are affected by a number of variables, such as age, phase of the menstrual cycle, and sexual stimulation.9.
Function of normal vaginal secretions
The well-established function of the vaginal fluid is to moisten the vaginal epithelium.10
Quantification and characteristics of normal secretions
Amount: Normal, physiologic discharge varies in amount from about one to 4 mL in 24 hrs11 and is heaviest at ovulation (day 14 of the 28 day menstrual cycle), and lightest on days 7 and 26.12
Table P-1: How normal discharge changes: relationship between the menstrual cycle hormones and vaginal secretions. 13
| Cycle Day | Estrogen | Progesterone | Secretions |
| 1-7 | Low | Very low | Menstrual flow begins and ends.Few secretions; dryness |
| 8-13 | Rises and peaks | Very low | Secretions increase |
| 14-16 | Drops sharply | Starts to rise | Ovulation; maximum clear mucus |
| 17-25 | Second small rise | Peaks | Secretions thicken and turn yellowish |
| 26-1 | Drops slowly | Drops slowly | Secretions diminish to low point |
Odor: Normal discharge has a mild, inoffensive odor (similar to sour milk) from the presence of lactobacilli. Its color ranges from white to clear and it may dry in a small white or yellow plaque on underwear. Discoloration of discharge, alone, should never be the criterion for diagnosis and treatment without evaluation first.
Other characteristics: Many women are unaware that vaginal discharge is a normal, physiologic phenomenon, and believe that they should be completely dry. They wear protective panty liners and may use feminine hygiene products in an attempt to do away with any discharge or odor, sometimes promoting irritation of the skin. In addition, many women are not aware that normal secretions can appear clumpy.
Upon noting clumpy, white discharge after a digital exam of the vagina, a woman immediately assumes yeast is present. Clinicians, too, often interpret normal, clumpy secretions as Candida. At mid-cycle secretions become abundant, clear, stretchable, like egg white, exhibiting the phenomenon of spinnbarkeit (German, ability to be spun).

Only such mucus appears to be able to be penetrated by sperm.
Hormonal changes: In postmenopausal women not on hormone replacement, whose estrogen states are low and stable, there is usually very little vaginal discharge. In some states of atrophy, women may complain of a sticky, yellow discharge. (Genitourinary syndrome of menopause). Low estrogen states causing changes on pH and wet mount may arise with lactation and other hormonal changes. Educating women about normal vaginal secretions is an integral part of vulvovaginal care.
Acidity of vaginal secretions: Because the vagina communicates with the outside, its epithelium is colonized by bacteria. Its weakly acidic environment, mostly commonly in the pH 3.5-4.5 range, sometimes slightly higher in different, asymptomatic, ethnic populations (up to 4.7-5.0)14 15 16 (See pH Values below) protects from bacterial overgrowth that might lead to infection. Women need to understand that the degree of acidity is not corrosive like sulfuric acid (pH 1) and that pH is controlled by endogenous vaginal factors (estrogen and lactobacilli), not by diet, hydration, or the use of probiotics. Douching with vinegar produces transient change in pH and is never recommended because of its risks to the upper genital tract.
For any clinician evaluating women for vulvovaginal complaints, the use of vaginal pH and a microscope is essential. An understanding of basic microscopy is mandated for obstetricians and gynecologists by the American College of Obstetrics and Gynecology and should be mandated for any clinicians who see patients with these complaints. (Principles of vaginal microscopy). Working without pH measurement and microscopy is the equivalent of working with one hand tied behind the back. Unfortunately, Hillier’s 2021 study showed that point-of-care tests including vaginal pH (15%), potassium hydroxide/whiff (21%), and wet mount microscopy (17%) were rarely performed. In their cross-referenced study, 47% received one or more inappropriate prescriptions, 34% were prescribed antibiotics and/or antifungals in the absence of bacterial vaginosis, tricomoniasis, or candidiasis, and return visits were more common for women treated empirically than for those not receiving treatment.17
There is no substitute for the information obtained from a wet mount: not only the presence or absence of Candida, bacterial vaginosis, and trichomonads, but also the state of estrogenization as represented by the vaginal maturation index (VMI) in which vaginal pH is correlated with microscopic exam for mature versus intermediate squamous cells and immature parabasals,18 and inflammation represented the number of white cells and parabasal cells. Alterations in background flora, normally dominated by lactobacilli, complete the diagnostic picture. These findings, combined with vulvar findings on exam, allow the pattern recognition essential to determine a diagnosis. Cultures and nucleic acid probes supplement this information, but do not substitute for it.
A light microscope, commonly found in physicians’ offices, is entirely adequate for diagnosis, (especially if a medical assistant can be designated to clean it on a regular basis). However, the use of a phase-contrast microscope is ideal because of the tremendous depth of detail that it supplies. Large and clear images facilitate identification of spores of Candida, the clue cells of bacterial vaginosis, and trichomonads. There is no other testing, other than a microscope, to identify vaginal inflammation through the recognition of parabasal cells from the vaginal wall epithelium. These may be present in active Candidiasis, desquamative inflammatory vaginitis (DIV), and with vaginal atrophy associated with menopause and lactation. (See Genitourinary syndrome of menopause.) With phase-contrast microscopy, the clinician can distinguish lymphocytes from neutrophils by their nuclei. Background flora are also distinct, enabling the identification of the dominance of lactobacilli morphotypes in a normal vaginal sample (research laboratory testing is necessary for confirmation of individual lactobacillus species), or their diminution with disease.
A phase-contrast microscope is expensive, costing several thousand dollars. However, considering the millions of dollars spent annually on multiple pediatric, primary care, gynecology and specialty visits in search of correct diagnosis, and the recompense through increased accuracy and immediacy of diagnosis and treatment, the investment, in our consideration, is worth it. A less expensive phase attachment for a light microscope is also available.
Deterrents to use of pH and microscopy in the USA include regulations from the Clinical Laboratory Improvement Amendment (CLIA) regarding point of care testing. Adherence to the CLIA regulations involves some basic staff training. PH test strips must be dated and checked regularly, and all involved staff must complete simple, computer based annual testing for competency. There is a learning curve required to master microscopy, but the payoff in microscopic pattern matching enabling confident, immediate discussion of the patient’s condition makes the investment of time economical in the end. (Principles of microscopy)
An excellent video on wet mount is available through the Brookside Institute. www.operationalmedicine.org/ed2/Video/wet_mount_video.htm
Note: A clinician can do a wet mount in the time it takes a patient to dress after an examination.
New understanding of the diverse microbiota of the human vagina has exploded old ideas about definitions of “normal” vaginal pH and “normal” vaginal bacterial populations and is opening up a world of possibility in the search for treatment of vaginal infections. Advances in this understanding burgeoned with the 2008 National Institute of Health (NIH) global study called The Human Microbiome Project (HMP).19 Further studies on the human vaginal microbiome specifically have given depth to what we need to know to care for patients.20 21 22
Information on this very important and interesting field of research is found in Annotation O: The Vaginal Microbiome.
As seen in the section on the Vaginal Microbiome, the balance (composition and abundance) of vaginal microbes directly affects pH levels. In addition, pH depends on estrogen effects on vaginal secretions. Estrogen stimulates glycogen deposition on the surface of vaginal epithelial cells. Both the epithelial cells and the microbial enzymes degrade glycogen to glucose. Lactobacilli then metabolize the glucose to lactic acid, resulting in a vaginal pH generally around <4.5. As pointed out, (see Vaginal Microbiome) there are subtle differences in vaginal pH between different ethnic groups in the USA (White (pH 4.2±0.30), Asian (pH 4.4±0.59), Black (pH 4.7±1.04),Hispanic (pH 5.0±074)) and between and within countries.23
A pH measurement, as it stands alone, is a non-specific finding. Therefore, pH has to be evaluated within a context. The normal pH of non-contaminated vaginal secretions in females across the age spectrum is fairly predictable. The presence of estrogen and of lactic acid-producing Lactobacilli in normal flora produce consistent acidic (pH <4.5) results in healthy, non-lactating or postpartum women of reproductive age, with the caveat that some ethnic groups may demonstrate slightly elevated normal pH levels. pH is also predictably altered in conditions of imbalance of normal flora, hormonal changes (low estrogen), or infection and can, therefore, be used in the process of diagnosis. Contaminants, such as soaps, gels, lubricants, blood, semen, urine and even water, can alter the pH. Therefore, pH can never be used without full systematic exam and microscopy in seeking a diagnosis.
The acidity or alkalinity of the vaginal secretions should be tested with pH test strips that come in a box with a color scale. The test strip should be applied with a dry, cotton-tipped applicator (Q-tip) to the vaginal sidewall in the lower third of the vagina to avoid secretions from the cervix, which may have an elevated pH (a normal finding in the cervix). Alternatively, the dry Q-tip may be applied directly to the vaginal sidewall and then to the pH test strip itself to see the color change. Once the secretion sample is placed on the color test strip, any color changes can be compared with the scale on the box. With the strips that we currently use, the original color of the strip is light green. The expected “normal” color change is to yellow in the case of a pH of <4.5. The range of colors is from yellow to light green to darker green to blueish green to dark blue and the range of pH measured is from 4.0 to 7.0. The color change is stable for two to five minutes at room temperature.

In healthy, reproductive age women who are not lactating, the spot on the test strip should change from the light green base to yellow (pH 4.0-4.5), again with the caveat related to women of differing ethnicities. In healthy, postmenopausal women not using estrogen, the pH will usually be elevated. This may or may not be associated with discomfort, abnormal discharge, or dyspareunia. (Genitourinary syndrome of menopause). Women who are lactating have decreased estrogen levels and concomitant elevated pH and thinning of the vaginal walls. This effect is more pronounced early in the lactation period.
An advantage of doing a vaginal pH immediately upon insertion of the speculum (or with a Q-tip alone if the woman cannot tolerate a speculum) is that a low pH of 4.0-4.5 precludes most bothersome vaginal conditions (bacterial vaginosis, trichomonas, desquamative inflammatory vaginitis, and atrophy). If you see a pH of 4.0-4.5, you can be reassuring to the woman right away. The only condition that will appear in an environment of any pH range is yeast. C. albicans actually prefers the low pH environment because of its positive association with estrogen. C. glabrata can grow in an environment with an elevated pH.
In most women, pH levels are directly correlated with the numbers of Lactobacilli found on wet prep, the next step in the diagnostic process (see wet mount below). Wet mount estimation of Lactobacilli, therefore, is a reliable indicator of vaginal pH.24 If a pH is elevated, crosschecking with the number of Lactobacilli seen can help to highlight contamination of the specimen versus the presence of pathology. (See wet mount below).
As stated above, the studies that show slightly elevated pH (to 5.0) in asymptomatic women of different ethnic groups also show different species of Lactobacilli being predominant. L. crispatus is more common in white and Asian groups, while L. invers is more dominant in black and Hispanic populations who are more likely to have normal pH values of 5.0. In addition, lactic acid-producing bacteria (some of them anaerobic) other than Lactobacilli may help to maintain the healthy vaginal ecosystem.25 Again, it appears that production of lactic acid is the key protective factor.
- Use cotton swab applicator to obtain secretions from the mid-portion of the vaginal sidewall.
- Avoid collecting the specimen from the cervix.
- Mix specimen with 0.5 cc saline suspension in test tube or paper cup.
- Saline should be fresh and at room temperature.
- Place a drop of the saline specimen on first slide.
- Place cover slip over specimen, avoiding air bubbles. Learn to avoid over-spill from your droplet which will soil the microscope lens.
- Perform microscopy within ten minutes.
- Add one drop of 10% KOH to another drop of saline mixture.
- Use a new slide or the other half of the first slide.
- Sniff for “fishy” amine odor that suggests BV or trichomonas.
- After the “whiff test,” place cover slip.
- Saline slide should be read first, then KOH.
Again, see the video resource by Michael Hughey, MD of Brookside Associates for learning how to perform the wet mount examination and whiff test. (Wet Mount)
- Put the slide on the stage.
- Rotate 10-x lens into place.
- Turn light on.
- Use coarse adjustment to focus.
- For contrast, lower condenser or adjust diaphragm to allow more or less light.
- Rotate 40-x lens into place.
- Increase light if necessary.
- Use fine adjustment knob to focus.
- Readjust diaphragm if necessary.
- Begin reading; review the entire slide.
- When using a phase contrast microscope, rotate to Phase III position to magnify.
- Vaginal Culture: Cultures for Candida or trichomonas (or rapid antigen and nucleic acid amplification tests), are indicated by clinical findings, or negative microscopy with clinical suspicion for these conditions. In one study, the sensitivity of microscopy for diagnosis of Candida and trichomoniasis was only 22 and 62 percent, respectively,26 and in another, microscopy failed to yield clear results 50% of the time, whatever the reason for that may be.27 Bacterial cultures of the vagina are not helpful and are rarely used by vulvovaginal experts. Vaginal bacterial cultures will always identify multiple strains of bacteria commonly found in and normal to the vagina, the skin, and the lower GI tract. Interpreting these as diagnostic of infection will lead to overuse of antibiotics and lack of efficacy of treatment. These do not need to be treated. This includes Group B Strep (GBS). This organism (GBS) is often recovered in non-pregnant patients, particularly in patients with desquamative vaginitis, but this laboratory finding neither establishes a diagnosis nor dictates treatment.28 The organism that provides key protection in the vaginal ecosystem is Lactobacillus.
- Cervical culture: Neisseria gonorrhoeae or Chlamydia trachomatis must always be considered in women with purulent vaginal discharge, fever, or lower abdominal pain. Any women with a history of high-risk behavior (new or multiple sexual partners), a symptomatic sexual partner, or an otherwise unexplained vaginal discharge that contains a high number of PMNs (polymorphonuclear leukocytes, more commonly called white blood cells) should be tested for the presence of these organisms. In addition, sexual behaviors that result in STI-related vulvovaginitis (e.g. trichomoniasis, herpes simplex virus) increase likelihood of coexisting STIs. The presence of high-risk behavior or any sexually transmitted disease requires screening for HIV, hepatitis B, and other STIs.
- Serologic tests: There are no serologic tests available for common causes of vaginitis. Serology, however, may be useful in the diagnosis of genital herpes, providing that evaluation be performed at least six weeks after the exposure. (Herpes simplex, the Atlas of Vulvar Disorders).
- Papanicolaou smear: The Pap test is an unreliable tool for diagnosing either bacterial vaginosis or trichomoniasis.29 30 When compared to Gram stain criteria for bacterial vaginosis, a Pap test has a sensitivity of 49% and specificity of 93%.31 In a symptomatic woman with bacterial vaginosis on a Pap test, a vaginal pH, amine test and wet mount should be performed; asymptomatic women do not need evaluation or treatment given that the diagnosis on Pap test is uncertain and it is unclear that asymptomatic, non-pregnant women with bacterial vaginosis benefit from treatment.32 For trichomoniasis, the Pap test has sensitivity similar to the wet mount but yields a false-positive rate of at least 8% with standard tests and 4% with liquid based cytology; thus, a diagnosis based on cytology can lead to an inaccurate diagnosis of a sexually transmitted infection. When feasible, in patients with trichomonas found on a Pap test, a wet mount and, if negative, a culture or one of the more sensitive nucelic acid amplification tests (NAATs) should be performed. If culture or NAAT is unavailable, the least expensive approach is to treat the patient with metronidazole. In populations with a low prevalence of trichomoniasis (5% or less), this approach may lead to an unnecessary treatment in more than 50% of cases. Optimal evaluation of the vagina includes pH, wet mount, and yeast culture done on several occasions since a single evaluation is inadequate to evaluate the dynamics of the vagina.
Other tests in the absence of the microscope: At times, patients must be evaluated without microscopy. In these cases, history, examination, and yeast culture for Candida are available. Elevated vaginal pH determines which patients need further testing for bacterial vaginosis and trichomoniasis. Point of care tests for trichomonas using DNA probe, (OSOM Trichomonas Rapid Test) or RNA probe (BD-Affirm), are available. Point of care tests for pH and amines (QuickVue Advance pH and Amines Test), G. vaginalis proline iminopeptidase activity (QuickVue Advance G. vaginalis test), and vaginal sialidases (OSOM BVBlue test), as well as RNA probe for Gardnerella (Affirm VP III) and PCR assays (BD Max Optima Panel and Aptima BV), as well as other laboratory-specific tests are FDA approved to aid in the diagnosis of BV. They are expensive and do not provide the information about inflammatory white blood cells and immature epithelial cells (parabasal cells) that come with microscopy.
The word “vaginitis” represents a spectrum of conditions that cause itching, burning, irritation, sometimes pain, abnormal discharge, and malodor.33 In the United States, the most common causes are bacterial vaginosis (22-50% of symptomatic women), vulvovaginal candidiasis (17-39%), and trichomoniasis (4-35%).34 Another 7-72% of women with vaginitis may remain undiagnosed,35 with symptoms caused by multiple other conditions: genitourinary syndrome of menopause (atrophy), desquamative inflammatory vaginitis, drug reactions, lichen planus, or vulvodynia. See Table P-6 below.
Vulvovaginal candidosis (VVC) (yeast vaginitis, Candida vulvovaginitis, candidiasis)
Introduction
VVC is defined as signs and symptoms of vulvovaginal inflammation in the presence of Candida species.36 Recurrent VVC (RVVC) is commonly defined as three or more proven cases in 12 months or at least three episodes unrelated to antibiotic use that occur within one year.37 Chronic VVC is defined as unremitting vulvovaginal inflammation causally associated with Candida.38
In 2016, the genome of Candida was sequenced, giving better information on the species’ features; but details of the intricate mechanism leading from colonization to infection are still not fully elucidated, and RVVC is not fully understood. One of the greatest hopes for further information from the genome is the development of truly fungicidal anti-fungal medications. Most currently available anti-fungals are fungistatic, decreasing the fungal load, but reliant on the vaginal host inflammatory reaction to clear up the infection. However, the recent development of “fungerp” triterpenoid medications which target fungal cell walls43 and novel azole medications which inhibit synthesis of fungal cell membranes and have very long half lives44 offer the promise of fungicidal action and greater clinical efficacy in the future. In addition, vaccines targeting various components of the fungal cell wall and elements essential for attachment and tissue invasion by Candida organisms are currently in early stages of development. The large variation in and plasticity of Candida species, and the body’s immune tolerance toward commensal fungi make this work challenging.45 Non-Candida albicans species are emerging pathogens and can also asymptomatically colonize human mucocutaneous surfaces, or produce symptomatic vaginitis or recurrent symptomatic vaginitis.46 See non-albicans Candida below.
Epidemiology of VVC
VVC is not a reportable disease, and therefore, the information on its incidence is incomplete and based on epidemiology studies that are often hampered by inaccuracies of diagnosis and/or the use of non-representative populations.47 Most, if not all women carry Candida in the vagina at some point of their lives, yet sometimes without symptoms of infection.48 Identification of vulvovaginal Candida is not necessarily indicative of disease since the definition of VVC requires both signs and symptoms of vulvovaginal inflammation in the presence of Candida species. But, in 70–75% of women, a diagnosis of VVC is made at least once during their childbearing years.49 It is estimated that 50% of initially infected women will suffer a second VVC event and 5–10% of all women will develop RVVC.50
The incidence of VVC in symptomatic women varies depending on the location in the world, as well as the populations studied.51 The highest incidences of Candida are reported by epidemiological studies made in African countries such as Nigeria,52 followed by Brazil53 then Australia.54
The lowest incidences are reported in the European countries of Greece (12.1%)55 and Italy (19.5%).56 In India, incidence of Candida ranges from 17.7 to 20.4%.57 58 59 Candida glabrata has been reported as the dominant species in studies from several African and Asian countries including Nigeria, Ghana, Turkey, India, and Lebanon.60 All of these epidemiological studies reported a higher incidence of VVC in women at reproductive age (20–40 years) than in women at menopause or in pre-pubertal girls.
Microbiology
There are more than 350 different species of Candida in nature. At least 13 Candida species cause infection in women. C. albicans is the most common.61
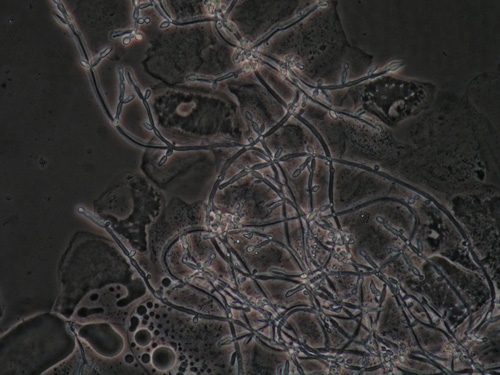
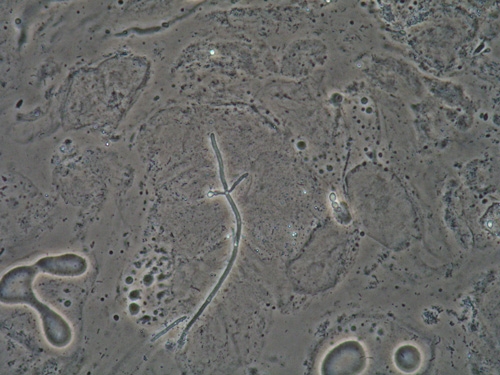
Other species of Candida are denoted as “non-albicans Candida,” with Candida glabrata the most common, C. tropicalis in second place, followed by C. parapsilosis and C. krusei. Culture is required to identify the species when Candida is recurrent or refractory.
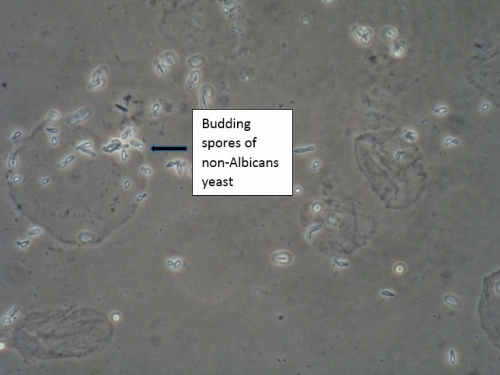
Typically, a single species is identified, but two or more species have been found in 1-10% of women with VVC.62 63 64 Most of these mixed infections are caused by an association between C. albicans and C. glabrata.65 66 67
Historically, 85–95% of Candida species identified in women with VVC were C. albicans.68 69 However, there are studies published during the 1990’s reporting an incidence of C. albicans below 85% and in some countries even below 50%. It has been suggested that the widespread and inappropriate use of anti-fungal treatments (self-medication and prolonged anti-fungal therapy) may lead to the selection of non-albicans species (such as C. glabrata), which are more resistant to the commonly used anti-fungal agents than is C. albicans.70 71
Lactobacilli may be protective through competition with Candida for nutrients, stearic interference with Candida adherence, and elaboration of hydrogen peroxide and inhibitory bacteriocins.72 Production of biosurfactants by lactobacillus species can prevent fungal adhesion to epithelial walls. Lactobacillus production of organic acids such as lactate and bacteriocins exert fungistatic effects. Saturation of epithelial adhesion sites and co-aggregation (adherence of vaginal bacteria to fungal organisms) by Lactobacillus species can prevent Candida adherence to epithelial walls. Lactobacilli can also reduce the expression of genes in Candida responsible for yeast adherence and hyphal formation. And, lactobacillus species alter the host immune response to attract granulocytes and promote host immune defense.73 However, at the present time, treatment with lactobacilli has not been effective in preventing antibiotic-related Candida.
Non-albicans Candida
Culture proven non-albicans Candida found in the vagina represents another challenge. Like Candida albicans, non-albicans Candida may be present, (although less commonly) as a commensal not responsible for perplexing vulvovaginal symptoms. It can also be the cause of isolated symptomatic vaginitis or recurrent symptomatic vaginitis, or act as a source of secondary infection in women with an epithelial disorder or with contact irritant use. Non-albicans yeast, most commonly C. glabrata, may be responsible for up to 33% of recurrent cases of candidosis.74 In fact, non-albicans species have been more commonly isolated among patients with RVVC than in women with sporadic VVC,75 possibly, as mentioned above, due to a higher anti-fungal exposure and widespread use of over the counter anti-mycotics among patients with RVVC.76
High percentages of non-albicans species causing VVC, mainly C. glabrata, have been also associated with increasing age,77 78 patients with uncontrolled diabetes,79 and HIV-infected women.80 These associations may occur from changes in patient physiology, estrogen balance, and decrease in immune functions.81
C. glabrata is predominantly found in northern Europe, the United States and Canada. C. krusei is seen more commonly in older white women. C. parapsilosis (Southern Europe, Asia and South America) is more common in transplant recipients and low birth weight infants. T. tropicalis (Asia-Pacific) occurs more commonly in association with pregnancy, neutropenia, and malignancy. C. dubliniensis is phenotypically similar to C. albicans, and may test as albicans. It is commonly found in HIV positive persons. C. auris is an emerging non-albicans species first seen in Japan in 2009 but currently spreading rapidly over multiple countries. Concerningly, this organism is difficult to diagnose by standard laboratory methods and has developed multi-drug resistance.82 83
Vaginitis caused by non-albicans species is clinically indistinguishable from that caused by C. albicans although signs and symptoms may be milder in some non-albicans candida infections.84 The use of non-azole anti-fungals, such as boric acid and flucytosine, has been shown to be effective in treating VVC caused by non-albicans species, especially C. glabrata,85 which demonstrates intrinsically low susceptibility to the azoles and the ability to develop high resistance to them.86
Pathogenesis of Candida
Access of Candida to the vagina
Candida gains entry to the vagina by crossing from the rectum over the perineum,87 but it has long been known that decreasing gastrointestinal carriage of Candida by oral administration of nystatin does not prevent recurrent symptomatic vaginal infection.88 A systematic review from an interdisciplinary and environmental medical point of view has been undertaken and failed to draw conclusions about the patho-genetic significance of intestinal Candida colonization.89
Candida infection may arise (less commonly) after sexual contact,90 or from relapse or persistence of the organism after previous Candida infection.
Colonization
In the vagina, Candida may live in harmony as a colonizer next to vaginal bacteria. The natural history of asymptomatic vaginal yeast carriage is unknown, but may exist for months or years.91
Vertical transmission from mother to infant during childbirth allows for early establishment of colonization of the oral cavity and the rectum with Candida, where its interaction with other resident microbiota and host immunity prevent transition from commensal organisms to pathogens. Extragenital colonization has been implicated as a reservoir of genital recolonization in patients with recurrent vulvovaginal candidiasis.92 At the vaginal level, epithelial cells under the influence of reproductive hormones93 provide a mechanical barrier, recognize and process antigens, secrete immune mediators and orchestrate the vaginal immune system in a delicate balance of protection against pathogens while allowing the immune tolerance necessary to accept semen and a developing fetus.94
Symptomatic candidal vaginitis (VVC)
Besides colonization, Candida has the ability to cause symptomatic vaginitis by transformation to an invasive pathogen. Candida may exist as a spore or a filament (hyphae or pseudohyphae). All forms are thought to play a role in the progression of vaginal infection in women.95 Transformation from colonization to infection involves a complex interaction of:
(1) host inflammatory responses (discussed below)
(2) Candida virulence factors.96 While it is usually assumed that the transition from asymptomatic colonization to symptomatic candidiasis occurs following a perturbation or loss of local defense mechanisms, this transition may also occur because of factors that enhance fungus virulence.97 These include:
- Morphologic plasticity: albicans is a polymorphic fungus that can reversibly transition between yeast, pseudohyphal and hyphal forms. The unicellular yeast form is commonly regarded as a harmless colonizer, but transition to the hyphal form allows the organism to adhere to and invade epithelial cells, resulting in extensive host cell damage. Host membranes are weakened by degradative enzymes secreted at the hyphal tip and pressure exerted by the elongating filament is then sufficient to penetrate the host cell.
- Phenotypic plasticity: albicans has the capacity to switch from a white phenotype producing smooth white colonies to gray and opaque phases which are less virulent. This switching between white and opaque phenotypes helps C. albicans evade host immune responses, as opaque cells are less susceptible to phagocytosis by macrophages and are able to evade killing by neutrophils.
- Genomic plasticity: albicans is capable of gross chromosomal rearrangement, aneuploidy, and loss of heterozygosity when exposed to different stresses. This allows the organism to adapt to a changing environment by changing the number of specific genes on a given chromosome. For example, amplification of two resistance genes on the left arm of chromosome 5 (ERG11 and TAC1) is associated with azole resistance.98
- Fungal products: albicans possesses multiple redundant pathogenic mechanisms for triggering inflammatory processes within the host. Candida virulence factors are genetically controlled, including an estradiol binding protein (EBP),99 multiple mechanisms for adherence of Candida to the epithelial cell prior to invasion, as well as ability for hyphal and biofilm formation.100 In addition, extracellular proteinases of C. albicans trigger immune responses locally and systemically.101 C.albicans has the ability to modulate phagocytosis by host neutrophils and macrophages, and endocytosis of C. albicans into epithelial cells both contributes to its invasiveness and stimulates phagocytic cells to produce inflammatory cytokines. In the non-albicans candida species, C. glabrata has highly efficient adhesion to various surfaces due to a range of adhesins, high stress resistance, and the shortest replication time of all Candida species. C. glabrata strains have an intrinsic resistance to azole antifungal medications, and mixed biofilms consisting of C. glabrata and C. albicans lead to more robust and complex structures and improve antifungal resistance.102.
- Drug resistance mechanisms: One of the main routes of resistance is the over-expression of efflux pump-related genes that results in decreased drug concentration within fungal cells (MDR1 genes in fluconazole resistance, and CDR1/CDR2 genes in resistance to all azoles). Overexpression of the ERG genes involved in ergosterol biosynthesis and biofilm formation also results in drug resistance among Candidal species.103
Since many Candida infections occur without a clear risk factor,104 it appears that focus on host inflammatory risk factors and Candida virulence factors is important.
At this time, new therapies are not available to address immune factors, but it is helpful to discuss with affected women that immune factors related to both the Candida organism and the vagina may cause their complex problems.
Recurrent vulvovaginal Candida (RVVC)
RVVC is usually defined as idiopathic, with no known predisposing factors, affecting up to 5% of all women who have a primary sporadic episode of VVC.105 106 Anti-fungal therapy is highly effective for isolated symptomatic infection but does not prevent recurrences. In fact, maintenance therapy with the efficacious anti-Candida drug fluconazole lengthens the time to recurrence but does not provide a long-term cure.107 There is now concern that repeated treatments might induce drug resistance,108 and result in an increased incidence of non-C. albicans, intrinsically resistant, species.109 110 111
Drug resistant Candida species have been a growing trend for the past decade, and multi-drug resistant Candida species are now being reported all over the world.112 Most non-albicans Candida species now have higher MIC’s (minimal inhibitory concentrations) for both prescribed and over the counter antifungal agents. Resistance is now common in non-albicans Candida species to the azole group of antifungal agents, and increased dose-dependent resistance is reported. In addition, organisms from patients with recurrent vulvovaginal candidiasis are more resistant to azole medications than those from uncomplicated vulvovaginal candida patients. 113
The pathogenesis of RVVC remains unknown. Many experts believe that RVVC results from relapse: DNA typing studies suggest that persistent Candida in the vagina from a previous infection invades again.114 The DNA of strains of Candida isolated from vaginal cultures of women before and after treatment for yeast vaginitis showed that 86% of the strains were identical.115 Some organisms persist within the vaginal vault, generally in numbers too small to be detected by conventional vaginal cultures, re-emerging to cause infection weeks or months later. Despite a 1993 report of a systemic immune deficit,116 there is now evidence that patients with RVVC are systemically immunocompetent. A local anti-Candida immune dysfunction in these subjects is suspected;117 for some forms of recurrences, the source could be genetic.118 119
A different perspective on Candidiasis turns away from the emphasis often focused on the estimated 5% of women with RVVC and the lack of demonstrated immune dysfunctions in these women. Instead, the focus might be on the 95% of women who, even after a first acute VVC attack, do not develop RVVC. These women may be colonized, but they remain largely disease-free for the rest of their lives, with only occasional, limited, and perfectly curable episodes during pregnancy or following some antibiotic therapy. This occurs despite more-than-frequent exposure to Candida and colonization from the gastrointestinal (GI) tract, suggesting that specific immunity may be induced by the initial, even sub-clinical episodes; this immunity may be boosted by commensalism and effectively prevents the onset of chronic infection.
Proponents of this perspective speculate that what is probably required for avoiding disease is control of the virulence traits of the fungus, which may allow the transition from commensal status to pathogenic status. Control of Candida virulence may be better achieved by mobilization of host immune responses through a vaccine.120 Multiple potential fungal targets for vaccine development have been explored between 1986 and the present, and two recombinant protein vaccines have progressed to Phase 1b and Phase 2a clinical trials (Schmidt et al 2012, and DeBernardis et al 2012). However, despite significant advances in immune and vaccine biology over time, a viable commercialized vaccine against fungal infections has yet to make it to market.121
Host inflammatory response factors
The host’s immune response is a crucial element of pathogenesis and host pathogen interaction.122 The immune system is separated into two branches: humoral immunity, for which the protective function of immunization arises from antibodies, complement proteins, and certain antimicrobial peptides located in the cell-free bodily fluid or serum (humor), and cellular immunity, for which the protective function of immunization is associated with cells: phagocytes, antigen-specific cytotoxic T-lymphocytes, and sometimes, the release of various cytokines in response to an antigen. It is established that cell wall constituents of Candida species have antigenic properties that can evoke both humoral and cellular host immune responses during the course of infection.
The mucosa of the female reproductive tract, with its tissue architecture, cervicovaginal secretions, and fluid, has been shown to contain humoral and cellular constituents of innate immunity, together with cell populations required for initiating, recruiting, and maintaining an efficient adaptive immune response.123
Cell mediated immune response
The cell-mediated immune response is clearly important in the host defense against Candida, as reflected by the higher prevalence of infections among individuals whose cellular immune systems are impaired. As discussed above, an early hypothesis was that the systemic cell-mediated immunity of the host was defective, but current consensus today is that women with RVVC have a local vaginal immune deficiency.124 This local immune deficiency has yet to be clearly defined.
The exact mechanisms of induction of cell-mediated immunity in VVC and RVVC have not yet been elucidated. T cells can be identified in large numbers in the human vagina. Their characteristics suggest migration to the vaginal epithelium in response to local antigenic stimuli and/or inflammatory chemokines.125 126
Neutrophils may also be implicated in host defense against Candida infection. Studies have shown that granulocyte colony-stimulating factor (G-CSF) increased neutrophil-mediated damage to pseudohyphae of C. albicans, perhaps in association with IFN-gamma.127 128 Yeast hyphal formation and expression of ECE1 which encodes for the hypha-expressed toxin candidalysin are the crucial virulence determinants driving neutrophil recruitment.129
Humoral immune response
The role of the humoral immune response in preventing disease progression during Candida infection has been unclear and controversial.130 131 Some of the host immune response to candidal infection may be modulated by elements of the vaginal microbiome. L. crispatus, one of the dominant members of the vaginal microbiome, can diminish C. albicans virulence and enhance local immune response of vaginal epithelial cells by modulating the immune cytokines and chemokines profile, i.e. upregulating IL-2, IL-6, IL-17, and downregulating IL-8. Lactobacilli can also inhibit biofilm formation by C. albicans. Also, low pH and bactericidal compounds secreted by Lactobacilli tend to suppress Candida overgrowth and its transition from a virulent yeast form to virulent hyphal form. Surfactant protein A, produced in the vaginal mucosa, provides host defense by opsonizing pathogens, altering levels of pro-inflammatory cytokines, stimulating oxidative burst by phagocytic cells and promoting differentiation of antigen presenting cells, thereby linking innate with adaptive immunity.132
However, work with polyclonal and monoclonal antibodies, as well as mannan-specific IgG antibodies which trigger both the classical and alternative complement pathways, 133 suggests that development of a vaccine or short-term protective antibodies may be feasible. Indeed, in both immunocompetent and immunocompromised mice, a vaccine against C. albicans conferred reduced fungal burden and improved survival.134
Host risk factors for Candida
Pregnancy and estrogen
Human Candidal vaginal infections occur almost exclusively during the reproductive years and are extremely rare in premenarchal and postmenopausal women.135 The prevalence and severity increase in the premenstrual week.136 It is well established that Candida vaginitis is more often seen in pregnant than in non-pregnant woman.137 Recurrence is more common and therapeutic response is reduced in pregnancy. Increased vaginal glycogen associated with high levels of reproductive hormones, becomes a carbon source for Candida. It is well known that estrogen increases adherence of yeast to vaginal cells, promoting germ tube formation, and that Candida possesses a cytosol binding system for estrogen.138 It is possible for a woman to be yeast free until the commencement of hormone replacement therapy at menopause.
Diabetes mellitus
Uncontrolled diabetes predisposes to yeast vaginitis, but candidiasis is not increased when the disease is well controlled.139 In glucose tolerance tests on women with recurrent candidiasis, there was no increased frequency of abnormal test results compatible with diabetes or pre-diabetes. Plasma glucose values were within the normal range, but were substantially higher than controls, suggesting that a diet high in refined sugars could contribute to the risk of candidiasis.140 Clear support for elimination of all carbohydrates does not exist in medical evidence. Women with type 2 diabetes appear prone to colonization and infection by non-albicans Candida species. Elevated plasma glucose levels and subsequent glucosuria induce proteins in Candida which favor yeast adherence. Treatment with sodium-glucose cotransporter 2 inhibitors may increase the risk for vulvovaginal candidiasis.141
Antibiotics
Estimates of the frequency of candidosis following antibiotic use ranges from 28% to 33%,142 thus, most women who receive antibiotics do not develop candidiasis. All antimicrobials are thought to cause candidosis by eliminating the protective bacterial flora, allowing overgrowth of Candida in the gastrointestinal tract, vagina, or both.143 Low numbers of lactobacilli have been found in vaginal cultures from some women with Candida vaginitis.144 But administration of lactobacillus (oral or vaginal) during and for four days after antibiotic therapy does not prevent post-antibiotic vulvovaginitis.145
Gastrointestinal tract
Although oral nystatin does not prevent vaginal Candida infections,146 there may be a link between symptomatic vaginal Candida infections and the gastrointestinal tract. In a study of both HIV infected and non-infected women, bowel movements appeared to correlate with fungal growth, as diarrhea was associated with an increase in vaginal fungal growth. Women who had diarrhea in the past 30 days were approximately twice as likely to have fungal growth than those who did not have diarrhea, which reached statistical significance in both groups.147
Immunosuppressive medications and disease
Use of corticosteroids is likely to be associated with recurrent Candida because of local suppression of immunity.148 Candida infections are also more common with use of immunosuppressive drugs, and with HIV infection.149
The long term administration of fluconazole in HIV positive individuals for prophylaxis against other fungal opportunistic infections has resulted in an increased prevalence of non-albicans Candida species in this population.150
Behavioral factors
Contraceptives
Contradictory results reported from different studies make the relationship between the OCP and Candida a controversial topic. Increased yeast colonization is reported in users of intrauterine contraceptive devices, sponges and diaphragms, and condoms, with and without spermicides.151 An extensive study of college students did not show an increase in the risk of symptomatic vulvovaginal candidosis in users of oral contraceptives, diaphragms, condoms, or spermicides.152
At least two studies have suggested that recurrent Candida vulvovaginitis in IUD user may be related to the ability of some species to attach to the IUD and form a biofilm rather than related to the IUD itself. Spermicides are not associated with Candida infection.153.
Sexual activity
Women who are not sexually active often develop vulvovaginal candidiasis; the incidence of the disease increases dramatically in the second decade of life, with the onset of sexual activity.154 Candida occurrence declines in women older than 40 years, but can resurface with use of estrogen after menopause.
The theory that recurrences are caused by re-infection of the woman by her male sexual partner has been suggested repeatedly, with some data suggesting that sexual transmission does occur.155 Candida species may be harbored in the male gastrointestinal tract, semen, urine, and oral cavity.156 Male colonization is associated with vaginal colonization, often with the same strain.157Reed et al158 studied sexual behaviors that were associated with recurrences of C. Albicans vulvovaginitis. Vaginitis was found to be unrelated to the presence of Candida in various body sites of the female or her male partner prior to the recurrence. Even Candida carriage in the vagina failed to predict subsequent symptomatic Candida vulvovaginitis. The following sexual risk factors for recurrent Candida vulvovaginitis were, however, identified: the female masturbating with saliva or cunnilingus from her partner, and the male masturbating with saliva or having had a younger age at first intercourse (reason unclear). They also found evidence that frequency/periodicity of sexual intercourse is associated with acute Candidal vaginitis frequency. Receptive oro-genital sexual intercourse consistently emerges as a risk factor for Candida.159 It is not clear whether this is related to transmission of the organism. Treatment of partners is not recommended unless they have symptoms.160 Occasionally, male partners of asymptomatic female carriers of Candida develop transient post-coital penile erythema and pruritus, suggesting inflammation from a hypersensitivity reaction. Women who exclusively have sex with women do not appear to have an increased risk of vulvovaginal infection.161
Other factors
Chemical contact, atopy, local allergy, or hypersensitivity reactions could alter the vaginal milieu and facilitate transformation from asymptomatic colonization to symptomatic vaginitis.162 Evidence is weak and conflicting regarding risk from sanitary pads and tight-fitting clothing.163
Both vaginal colonization and transition to infection have been linked to certain host genetic factors, including mannose-binding lectin polymorphisms, ABO-Lewis non-secretory blood group phenotype, and Y238X polymorphism in the Dectin-1 gene.164
Clinical manifestations
The clinical spectrum of symptomatic vaginitis varies from an acute, exuberant, exudative form with copious, white, vaginal discharge with little to no odor, and large numbers of germinated yeast cells, to the absence of discharge, fewer organisms, but the same severe pruritus. Clinical signs and symptoms in infections caused by different Candida species are a source of debate; C. glabrata and C. parapsilosis tend to be associated with milder, often absent symptoms.165 Vulvar pruritus is the dominant feature of candidal vulvovaginitis. Women may also complain of dysuria (typically perceived to be external or vulvar rather than urethral), soreness, irritation, and dyspareunia. There is often little or no discharge; but the typical white and clumpy (curd-like) secretions may be seen. Symptoms are often worse in the week prior to menses.
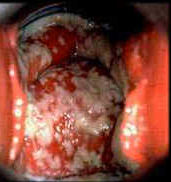
However, the secretions may also be thin and loose, indistinguishable from the discharge of other types of vaginitis.
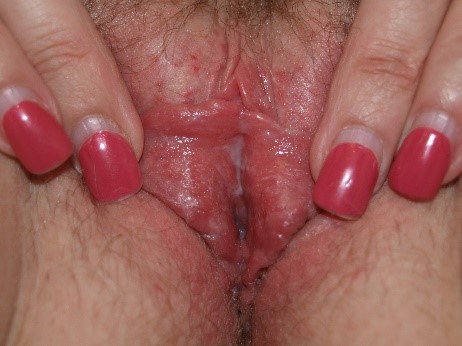
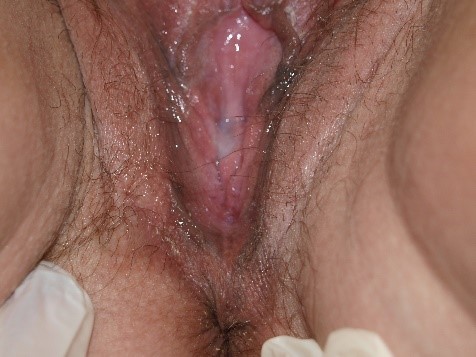
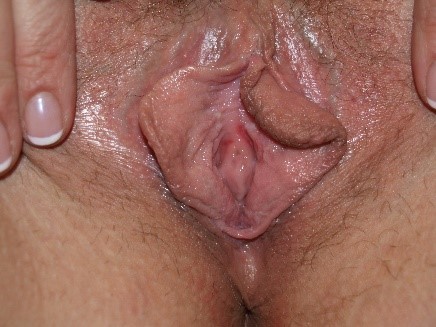
Diagnosis of Candida by simple inspection of vaginal secretions is not possible. Some patients, primarily those with C. glabrata infection, have little discharge and often only erythema on vaginal examination.166 Evidence of excoriation may be present. Candida albicans is also a frequent cause of vulvar fissuring.

Diagnosis of Candida
Reliable diagnosis of Candida cannot be made on history and physical examination alone. Diagnosis requires:
- visualization of blastospores or pseudohyphae on saline or 10% KOH microscopy or
- a positive culture in a symptomatic woman. A culture is of no value if the woman has recently used anti-fungal treatment. Repeating the culture if a woman continues to be symptomatic after treatment is important. Some experts do not recommend yeast cultures if microscopy is negative. On the other hand, microscopy skills vary widely, artifacts (hair and fibers, overlapping cells) abound, and the cost of culture has come down. A negative culture in the presence of vulvovaginal symptoms educates the woman that Candida albicans is not the reason for her discomfort, and encourages the clinician to look for other explanations.
In addition to vaginal yeast culture, there are a number of newer diagnostic technologies on the market. They vary in sensitivity, specificity, which species of Candida are tested for and reported, and availability in different health care settings. These point-of-care tests are considerably more expensive than in-office microscopy, but may be helpful in the setting of recurrent symptoms or low fungal loads.167
- DNA Probes:
-
-
- Affirm VP III (BD): Probe for Candida, Gardnerella, and Trichomonas. 58% sensitive, 100% specific. Detects “Candida species” without reporting individual speciation.
-
- Polymerase Chain Reaction (PCR) Assays:
-
-
- BD Max Vaginitis Panel (BD): 90.7% sensitive, 93.6% specific. Detects Candida Group (C. albicans, C. tropicalis, C. parapsilosis, C. dubliniensis), C. glabrata, and C. krusei. FDA cleared.
- SureSwab Candidiasis (Quest Diagnostics)
-
- Nucleic Acid Amplification Tests (NAATs):
-
- NuSwab (LabCorp): 97.7% sensitive, 93.2% specific. Detects C. albicans and C. glabrata.
- SureSwab Advanced Candida Vaginitis (CV), TMA (Quest Diagnostics): Employs transcription mediated amplification (TMA). Reports C. albicans, C. parapsilosis, C. tropicalis, C. dubliniensis, and C. glabrata.
In addition, there is an emerging market for at-home testing for vaginitis. These tests range from simple pH testing for bacterial vaginosis, to immunoassays and NAATs which test for Candida and other vaginal and cervical pathogens. Some platforms report results at the point of testing, and some need to be sent out and will report results within 2 to 5 days. There is currently little to nothing in the medical literature evaluating the sensitivity, specificity, and accuracy of these tests, and their cost can be substantial, but some of them offer the convenience and privacy of home self-collection.
Pap smear is positive in 25 percent of patients with culture positive, symptomatic vulvovaginal candidiasis. 168 But since the cells evaluated on a Pap smear are from the cervix, and are not affected by Candida vaginitis, the Pap smear is not a sensitive test.169 If Candida is found on a Pap smear of an asymptomatic woman, treatment is not required.
Most women with RVVC are HIV negative. Only women with RVVC who have risk factors for HIV infection should be tested.170
It is well established that self-diagnosis is frequently inaccurate.171 In a study that administered a questionnaire to 600 women to assess their knowledge of the symptoms and signs of vulvovaginal candidiasis (and other infections) after reading classic case scenarios, only 11 percent of women without a previous diagnosis of vulvovaginal candidiasis correctly diagnosed this infection. Women who had had a prior episode were more often correct (35%) but were likely to use over-the-counter drugs inappropriately to treat other, potentially more serious, gynecologic disorders. In another report, the actual diagnoses in 95 women who self-diagnosed vulvovaginal candidiasis were: vulvovaginal candidiasis (34%), bacterial vaginosis (19%), mixed vaginitis (21%), normal flora (14%) Trichomonas vaginitis (2%), and other (11%). Women with a previous episode of vulvovaginitis candidiasis and those who read the package insert for their over-the-counter medication were not more accurate in making a diagnosis than other women.172.
Diagnosis may be confounded when co-existent causes of symptoms (itching e.g.) are present, such as non-albicans Candida and a skin disorder, or Candida and vulvodynia. Consideration of the multiple other sources of vulvovaginal irritation prior to treatment of the culture positive Candida is important. (Consult Table P-6). Confirm whether the vulvovaginal epithelium appears quiet or inflamed. Lack of erythema or lesions suggests that inflammation from yeast is not present. Conversely, presence of erythema and/or alterations in skin texture or integrity may be signs of either Candida or a vulvar skin condition, irritant use, or other conditions, as listed in the table. Loss of vaginal architecture requires consideration of lichen sclerosus and/or biopsy.
Many vulvovaginal experts do not treat a positive culture for non-albicans Candida unless they are convinced that symptoms arise from the Candida and not another source. Watching and waiting, after explanation to the patient that the non-albicans yeast may be an innocent bystander, may show that symptoms are not significant and culture stays positive in a patient whose irritative symptoms do not add up to a diagnosis of Candida vulvovaginitis. It is possible that the patient has vulvodynia with irritative symptoms suggesting yeast if they have been present repeatedly over time when cultures have been negative. If the conditions in Table P-6 are eliminated, vulvodynia is the diagnosis.
Over a twenty five years ago, Candida albicans was classified as uncomplicated or complicated.173 The classification has been accepted internationally.174
Table P-5: Uncomplicated and complicated Candidiasis (Candidosis/Candida vulvovaginitis)175
| Uncomplicated candida vulvovaginitis | Complicated candida vulvovaginitis |
|---|---|
| Sporadic or infrequent episodes | Recurrent episodes (three or more per year) |
| Mild to moderate symptoms or findings | Severe symptoms or findings |
| Suspected Candida albicans infection | Suspected or proved non-albicans Candida infection |
| Non-pregnant women without medical complications | Women with diabetes, severe medical illness, immunosuppression, pregnancy, other vulvovaginal conditions |
Differential diagnosis
As discussed under Diagnosis, many women presenting with irritative vulvovaginal complaints are initially treated for Candida. Table P-6 shows the lengthy differential for pruritic and irritative vulvovaginal complaints. It is also important to recognize that Candida can occur concomitant with, or as a secondary infection with many of these conditions.
Table P-6: Differential diagnosis of irritative vulvar symptoms
| vaginitis (Candida albicans, non-Candida, BV, trichomoniasis) |
| sexually transmitted infections |
| desquamative inflammatory vaginitis (DIV) |
| contact irritants, allergens |
| seminal plasma allergy |
| loss of estrogen at any age after puberty |
| vulvar intraepithelial neoplasia (VIN) |
| dermatitis (eczema), dermatosis |
| lesions, ulcers, erosions, fissures, papules, pustules |
| systemic disease, e.g. Sjögren, Crohn |
| drug reaction |
| pain syndromes and hyperpathic itching |
| squamous cell carcinoma |
Treatment of uncomplicated candida vulvovaginitis
Treatment of uncomplicated candida vulvovaginitis can be any oral or vaginal azole. There are no significant differences in efficacy among topical and systemic azoles (cure rates >80 percent for uncomplicated vulvovaginal candidiasis).176 There are, however differences in the base ingredients. Miconazole, terconazole and butoconazole all contain propylene glycol to which many women are sensitive. They will tell you they had an “allergic” reaction to one of these topical drugs, meaning they had immediate contact irritation with redness and burning. Clotrimazole has no propylene glycol and is less likely to cause irritation. It is our preferred topical azole choice.
A single dose of fluconazole 150 mg orally is effective and usually preferred by women to the topical treatments, although it may take up to 48 hours before symptoms abate. Fluconazole interacts with some other drugs so careful review of medications being taken should be reviewed.177 178 In addition, in a nationwide cohort study in Denmark, use of oral fluconazole in pregnancy was associated with a statistically significant increased risk of spontaneous abortion compared with risk among unexposed women and women with topical azole exposure in pregnancy. Please see the Pregnancy section under Treatment for further information regarding use of fluconazole in pregnancy.
In women susceptible to symptomatic yeast infections with antibiotic therapy, a dose of fluconazole (150 mg orally) at the start and end of antibiotic therapy may prevent post-antibiotic vulvovaginitis.
Uncomplicated infections usually respond to treatment within a couple of days. There is no medical contraindication to sexual intercourse during treatment, but it may be uncomfortable until inflammation improves. Treatment of sexual partners is not indicated.180.
Table P-7: Treatment for uncomplicated Candidiasis (Candidosis/Candida vulvovaginitis) (*= most frequently used regimen)
| Intravaginal agents for uncomplicated Candida vulvovaginitis | Oral agents for uncomplicated Candida vulvovaginitis |
| Butoconazole 2% cream 5 g intravaginally for 3 days* | Fluconazole 150 mg oral tablet, one tablet in single dose (avoid in pregnancy) |
| Butoconazole 2% cream 5 g (Butaconazole1-sustained release), single intravaginal application | Ibrexafungerp two 150 mg tablets (300 mg) taken twice, 12 hours apart (600 mg total) |
| Clotrimazole 1% cream 5 g intravaginally for 7–14 days* | |
| Clotrimazole 2% cream 5 g intravaginally daily for 3 days | |
| Clotrimazole 100 mg vaginal tablet for 7 days | |
| Clotrimazole 100 mg vaginal tablet, two tablets for 3 days | |
| Miconazole 2% cream 5 g intravaginally for 7 days* | |
| Miconazole 4% cream 5 grams intravaginally daily for 3 days | |
| Miconazole 100 mg vaginal suppository, one suppository for 7 days* | |
| Miconazole 200 mg vaginal suppository, one suppository for 3 days* | |
| Miconazole 1,200 mg vaginal suppository, one suppository for 1 day* | |
| Nystatin 100,000-unit vaginal tablet, one tablet for 14 days | |
| Tioconazole 6.5% ointment 5 g intravaginally in a single application* | |
| Terconazole 0.4% cream 5 g intravaginally for 7 days | |
| Terconazole 0.8% cream 5 g intravaginally for 3 days | |
| Terconazole 80 mg vaginal suppository, one suppository for 3 days |
Treatment of complicated candida vulvovaginitis
Women with severe inflammation or factors associated with complicated Candida vulvovaginitis are unlikely to respond to short courses of topical anti-fungal drugs or a single dose of fluconazole. Observational series have reported that these patients require 7 to 14 days of topical azole therapy, rather than a one to three day course.181 A randomized trial demonstrated that two doses of oral therapy 72 hours apart were more effective than one dose.182 Comparative trials of topical versus oral treatment of complicated infection have not been performed. Since the oral anti-fungals can take 48 hours or more to reduce inflammation and promote improvement of symptoms, a topical anti-fungal or a low potency topical corticosteroid applied to the vulva (in addition to the oral medication) for 48 hours can be helpful.
Table P-8: Treatment of complicated candida vulvovaginitis (intractable Candida Albicans, non-pregnant patient)
| Intravaginal agents for complicated Candida vulvovaginitis | Oral agents for complicated Candida vulvovaginitis |
|---|---|
| Any intravaginal azole used for 7-14 days | Fluconazole 150 mg orally x 1 and again in 72 hours; in severe cases, may be used a third time. (Avoid in pregnancy). |
| Ibrexafungerp 150 mg tablets by mouth twice daily. The optimal duration of therapy is unknown. Avoid in pregnancy. |
Treatment of recurrent Candida vulvovaginitis (RVVC)
Recurrent vulvovaginal candidiasis is defined as three or more episodes of symptomatic infection within one year. Vaginal cultures should always be obtained to confirm the diagnosis and to identify less common Candida species. In most people, recurrent disease is due to relapse from a persistent vaginal reservoir of organisms or endogenous reinfection with an identical strain of susceptible C. albicans, but 10-20% of recurrent infections are from a different candida strain, often a non-albicans species. Some patients exhibit an immune hyper-reactivity to Candida due to mutations and genetic polymorphisms of innate immune genes which alter the vaginal mucosal immune response to Candida challenge.
Treatment strategy for recurrent infections includes an induction phase followed by maintenance:
Induction: Any topical agent vaginally X 7-14 nights, or oral fluconazole 150 mg orally every 72 hours for three doses, or Itraconazole 200 mg orally twice daily X 3 days
Topical induction regimens include:
- Clotrimazole 1% vaginal cream for 7-14 nights
- Miconazole 2% vaginal cream for 7-14 nights
- Miconazole 100 mg vaginal suppository for 7-14 nights
- Clotrimazole 2% vaginal cream for 3-7 nights
- Miconazole 4% vaginal cream for 3-7 nights
- Miconazole 1200 mg vaginal suppository once
- Miconazole 200 mg vaginal suppositories for 3-7 nights
- Tioconazole 6.5% ointment for one night
- Terconazole 0.4% vaginal cream for 7-14 nights
- Terconazole 0.8% vaginal cream for 3-7 nights
- Terconazole 89 mg vaginal suppository for 3-7 nights
- Butoconazole 2% vaginal cream single dose
Maintenance: Fluconazole 150 mg orally once per week X 6 months, or Itraconazole 100-200 mg orally twice weekly for 6 months, or miconazole 2% vaginal cream 5 gms vaginally twice weekly X 6 months, or miconazole 1200 mg vaginal suppository once weekly X 6 months, or clotrimazole 1% vaginal cream 1 applicator twice weekly X 6 months, or terconazole 0.4% vaginal cream twice weekly X 6 months.
After 6 months of suppressive therapy, a trial of drug discontinuation is recommended. Some patients will enjoy a prolonged remission after maintenance, but up to 55% will relapse and will need longer term suppression, often for additional months or years. Due to the safety profile and low plasma concentrations of once-weekly 150 mg fluconazole, most experts do not recommend laboratory monitoring during maintenance therapy.183
Oteseconazole was FDA approved in 2022 as a new oral azole antifungal to reduce the incidence of recurrent candida vulvovaginitis (RVVC). This is the first drug to be approved in the US specifically for treatment of RVVC. Oteseconazole is active in vitro against most isolates of C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. lusitaniae and C. dubliniensis. It is active against fluconazole-resistant isolates. Its mechanism of action is the same as other azoles, inhibition of fungal sterol 14⍺-demethylase (CYP51), an enzyme which aids in synthesis of fungal cell membranes. Adverse effects include headache and nausea. The drug has a 128 day half-life, and persists in tissues for many months. In two clinical trials oteseconazole recipients had fewer RVVC episodes and experienced a greater recurrence-free time interval compared to patients receiving placebo during a 48 week study period. Ocular abnormalities were observed in the offspring of pregnant rats given oteseconazole, and the drug is contraindicated in females of reproductive potential (including those using effective contraception) and in pregnant and lactating women.The drug should be taken with food to aid absorption and swallowed whole (not chewed, crushed, dissolved or opened. It is not recommended in patients with renal impairment or severe hepatic impairment. The medication comes in a blister pack to facilitate dosing. The cost of an 18 capsule course of therapy in 2022 was $2700.184.
Single Drug RVVC Treatment with Oteconazole: 600 mg orally on day 1, 450 mg orally on day 2, followed by 150 mg orally once weekly for 11 weeks, starting on day 14.
Dual Drug RVVC Treatment: Induction with fluconazole 150 mg orally on days 1, 4, and 7. Then suppressive therapy with oteseconazole 150 mg orally once a day for 7 days on days 14 through 20, followed by oteseconazole 150 mg orally once per week. 185
Treatment of non-albicans yeast
Non-albicans organisms commonly fail treatment with azoles (around 50 percent). Fluconazole resistance has been reported in some case studies of immunosuppression and systemic cases and is not recommended as first line therapy for non-albicans yeast.186 Treatment with terconazole 0.4% cream for seven days is reported to have a 56% mycological cure at one month,187 but this may be an over-estimate since the study did not track patients who refused terconazole because it had not worked before. Moderate success (65 to 70 percent) in women infected with non-albicans Candida with intravaginal boric acid (600 mg capsule inserted vaginally once daily at night for two weeks).188. But boric acid capsules used vaginally may be irritating. They can be fatal if swallowed, and there are no safety data on long term use. Most appropriate use currently appears to be for proven azole-resistant, non-albicans Candida.189
A 90 percent cure has been achieved with intravaginal flucytosine cream 10% (5 g vaginally nightly for two weeks),190 but the expense of the medication is prohibitive for some. Amphotericin B suppositories (50 mg intravaginally nightly for 14 days) are also of value in the treatment of non-albicans yeast. Boric acid capsules, amphotericin, and flucytosine cream are not available commercially and must be made by a compounding pharmacy.
Table P-9: Treatment of non-albicans yeast (not always necessary: rule out other causes to be sure non-albicans candida may be causing symptoms)
| Intravaginal agents for culture-proven non-albicans Candida in symptomatic women | Oral agents for culture-proven non-albicans Candida in symptomatic women |
|---|---|
| Compounded boric acid suppositories, 600 mg intravaginally, nightly x 14 days | Fluconazole is usually not effective in treating non-albicans candidiasis. |
| If not effective, follow a second treatment with boric acid intravaginally, 600 mg nightly x 14 days with nystatin cream: 100,000 units vaginally nightly for 21 days | Ibrexafungerp 300 mg orally every 12 hours X 2 doses. |
| If not effective, follow the second treatment with compounded flucytosine cream 10%, 5 grams intravaginally for 14 days, or Amphotericin B compounded suppositories, 50 mg intravaginally nightly for 14 days or or amphotericin B cream 3-4%, 5 gms vaginally at bedtime X 14 days | For C. krusei: Itraconazole 200 mg orally twice daily X 7-14 days. |
| Gentian Violet 1% solution: soak a tampon in 0.5 ml, place vaginally x 3-4 hours twice daily for up to 12 days. Will stain clothing. | Ketoconazole 400 mg orally daily X 7-14 days |
| For C. krusei: (typically resistant to oral fluconazole and flucytosine) | |
| Topical clotrimazole, miconazole, or terconazole vaginally X 7-14 days. | |
| Boric Acid 600 mg vaginal capsules or suppositories at bedtime X 14 nights | |
| Amphotericin B 3-4% vaginal cream or 100 mg vaginal suppositories at bedtime X 14 | |
| For C. parapsilosis, C. tropicalis, C. lusitaniae and C. kefyr: sensitive to azoles, which are first choice of therapy | |
| For C. dubliniensis: Boric Acid 600 mg vaginal capsules or suppositories at bedtime X 14 nights | |
| For Saccharomyces cerevisiae: resistant to fluconazole. Treat with topical clotrimazole or miconazole first. If not successful, use boric acid. |
Pregnancy
Treatment of Candida in pregnant women often fails to clear the pathogen and is usually indicated only for relief of symptoms. There is no association with adverse outcomes from vaginal candidiasis in pregnancy.191 Any of the topical azoles used vaginally for seven days is appropriate. Vaginal nystatin is another option, for 7 to 14 days. Avoid fluconazole, flucytosine, boric acid, amphotericin B and itraconazole in pregnancy.
There are several published case reports of birth defects in infants whose mothers were treated with high-dose fluconazole (400-800 mg/day) for serious and life-threatening fungal infections during most or all of the first trimester.192 193 The features seen in these infants include cranio-facial abnormalities, bony abnormalities, muscle weakness and joint deformities, and congenital heart disease. Based on this information, the pregnancy category for fluconazole indications (other than vaginal candidiasis) has been changed from category C to category D. 194 The pregnancy category for a single dose of fluconazole 150 mg to treat vaginal candidiasis has not changed and remains category C. Pregnancy category D means there is positive evidence of human fetal risk based on human data but the potential benefits from use of the drug in pregnant women with serious or life-threatening conditions may be acceptable despite its risks.
A US based population cohort study found that compared with topical azoles, oral fluconazole use in early pregnancy was associated with an increased risk of musculoskeletal malformations but not conotruncal malformations or oral clefts. This resulted in 12 additional incidents per 10,000 pregnancies, for a relative risk of 1.30.195. And in a 2020 meta-analysis of 9 current studies between 2000 and 2014, first trimester exposure to fluconazole was associated with an increased prevalence of heart defects in offspring in both low dose (OR 1.95) and any dose (OR 1.79) cohorts. No association was found between gestational exposure and increased risk of spontaneous abortion or stillbirth. The author’s conclusion was that fluconazole should be regarded as a human teratogen and should be prescribed with caution in pregnant women and women of childbearing potential.196
Alternative treatments
There is no evidence from randomized trials that garlic, tea tree oil, yogurt, or other products containing live lactobacillus species, or douching is effective treatment for or prevention of vulvovaginitis due to Candida albicans.
Gentian violet
Formerly a frequent treatment for Candida, it has fallen into disuse as treatment technology for yeast evolved. It is reported to affect chitin production on the yeast’s cell wall, and is suggested for combination with topical antifungal cream at night with repeat in seven days.197 Vulvovaginal irritation and staining of clothing are problems associated with painting the vagina with gentian violet.Safety of gentian violet has come into question. One study in mice demonstrated dose-related carcinogenic potential at several different organ sites. 198 Gentian violet has also been linked to cancer in the digestive tract of other animals. 199 It is generally considered safe for use on children and breastfeeding mothers. It has even been applied to the mouth and lips of premature infants, and has a long history of safe use. La Leche League recommends gentian violet for thrush on the nipple.200 However, in large quantities, gentian violet may lead to ulceration of a baby’s mouth and throat and is linked with mouth cancer. 201
Bacterial vaginosis
Introduction
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge and malodor in reproductive age women and represents up to 50% of all cases of “vaginitis.”202 BV is understood to be a “dysbiosis,” that is, an alteration or imbalance in the beneficial homeostasis of normal flora in the vaginal micobiome, rather than an inflammatory infection.203 Symptoms range from none at all to significant discharge and odor. Studies have shown an increased risk of sexually transmitted infections and preterm labor in the setting of symptomatic BV.
Epidemiology
In 2019, a systematic review and meta-analysis of global BV cases among reproductive-aged women found a 23-29% prevalence across all regions.204
Racial, ethnic, and geographical variations exist but the reasons are unclear. A 2013 global epidemiological study showed differences in populations worldwide with higher rates of BV in women from South and East Africa (68% in Mozambique, 51% in Lesotho, 44% in Kenya, 37% in Gambia) than in women from West Africa (7% in Burkina Faso). Rates in Norway were 24%, Turkey 23%, and Poland 19%. In Southeast Asia, Australia, New Zealand, and Indonesia, rates are usually greater than 30%. Prevalence is lower in Latin American and the Caribbean except in Jamaica. The prevalence in Australia was determined to be 4.7% in 2008.205 In a 2019 study which mostly corroborated these numbers, North American black and Hispanic women had significantly higher (33% and 31% respectively) prevalence of BV compared with other racial groups (white 23%, Asian 11% (p<0.01)).206 “This disparity persisted when study populations were classified as majority (>50%) black women or majority non-black women, with approximately 2-fold higher prevalence among majority black study populations (46.5%; 95% CI, 37.5–55.6 vs. 21.3%; 95% CI, 16.7–26.3; P < 0.001).”207
In this same study, among pregnant women, BV prevalence ranged from 11.7% in South Asia (95% CI, 9.0–14.7) to 33.2% in Latin America and the Caribbean (95% CI, 14.8–54.7). Within the United States, the prevalence of BV in pregnancy was highest among black (49.0%; 95% CI, 40.2–57.8) and Hispanic women (42.7%; 95% CI, 36.4–49.1) and lowest among Asian (20.3%; 95% CI, 5.4–41.2) and white women (19.9%; 95% CI, 8.0–35.5)208
Etiology
The cause of BV is still unknown, but the clinical manifestations of the disorder are well documented. As we learn more about the normal vaginal microbiome of reproductive age women, we also learn about the changes in microbiota that culminate in these distressing symptoms. Researchers are looking into the vaginal microbiome for both a cause and a cure. Despite subtle variations between ethnically and geographically diverse groups of people, the normal vaginal microbiome is predominantly populated by hydrogen peroxide and lactic acid-producing “good bacteria,” Lactobacillus species.209 These bacteria create the low pH environment (usually 4.0 to 4.5, with some groups normal at 5.0) present in healthy vaginas. When Lactobacilli predominate, there are fewer species in the normal microbiome. By contrast, BV is described as a disturbed microbiota dominated by increased numbers of gram-positive cocci and gram-negative bacilli: mixed anaerobes which cause the pH to be elevated.210 Concentrations of bacteria increase 100 to 1,000-fold in women with BV.211
The type of dominant bacteria in BV can vary. A 2011 study found the phylotypes most likely to correlate with a high Nugent score (the test used to identify BV in research settings) were Aerococcus, Anaeroglobus, Anaerotruncus, Atopobium, Coriobacteriaceae.’Dialister, Eggerthella, Gardnerella, Gemella, Megasphaera, Mobiluncus, Parvimonas, Peptoiphilus, Prevotella, Porphyomonas, Prevotellaceae_1, Prevotellaceae_2, Ruminococcaceae, and Snethia .212 In 2005, researchers found that women with BV had a mean of 12.6 bacterial phylotypes compared with 3.3 in controls. Three new bacteria in the Clostridiales order were found and named “bacterial vaginosis-associated bacteria” (BVAB).213 Prevotella, previously thought to be rare in vaginal isolates, was found in 68.5% of cases of BV, with abundance ranging from a few percent to 45%. Prevotella spp. have been shown to positively affect the growth of Gardnerella vaginalis and Peptostreptococcus anaerobius by producing key nutrients for these species, such as ammonia and amino acids. Since both of these species have been linked to bacterial vaginosis, the wide distribution of Prevotella spp. in the vaginal microbiota might be a factor that facilitates BV.214
As the Lactobacillus population decreases, pH rises, overgrowth of anaerobes occurs, and large amounts of proteolytic carboxyase enzymes are produced. These are capable of breaking down vaginal peptides into volatile and malodorous amines that are associated with increased transudation from the vaginal epithelium and exfoliation of squamous epithelial cells. While not known to be the causative agent for BV, Gardnerella vaginalis appears to be instrumental in providing a matrix (or scaffolding) for development of a treatment-resistant vaginal biofilm.215
Current thinking places the G. vaginalis biofilm at the heart of the initiation and progression of BV.216 Epithelial cells dotted with bacteria exfoliate from the biofilm, creating the “clue cells” that are diagnostic of BV.217 (It is important to remember that, while Gardnerella was detected in culture samples from nearly all symptomatic women with bacteral vaginosis, it is also found in approximately 50% of the vaginal microflora of healthy women. It is possible that there are species of Garnerella that are pathogenic and others that are not.)218219 Biofilms may be related to persistence or recurrence of bacterial vaginosis, as organisms in the biofilm state are less susceptible to antibiotic therapy.
Extracellular DNA (eDNA) has been found to also play a role in building the stable matrix of the BV biofilm. DNase has been used, in more than one in vitro study (and partially in an in vivo study), to both prevent new biofilm formation and also to liberate G. vaginalis from existing biofilms, reducing the density of the biofilm and possibly making it more reponsive to antimicrobials.220
Although there is no clinical evidence of inflammation with BV (no WBCs on wet mount), some research points to BV as a pro-inflammatory condition with specific BV-associated bacteria eliciting the production of pro-inflammatory cytokines.221
The prevalence of dysbiosis of the VMB and BV in racial and ethnic minority groups worldwide has prompted an examination of the impact of psychosocial stress on the microbiome. In the vagina, by binding to glucocorticoid receptors in the vaginal wall, stress-induced cortisol is shown to inhibit the deposition of glycogen in a manner which restricts the Lactobacillus population, leading to an increase in proinflammatory cytokines and chemokines and mucosal immunosuppression. 222 However, whether or not this inflammatory response is solely protective or solely harmful is a subject for debate as it is proposed that people who experience higher levels of stress may have adapted nLDVMs that are able to create acidic environments without reliance upon Lactobacillus.223
Risk factors
Risk factors for BV include multiple or new sexual partners, douching,224 (but not other personal hygienic behaviors), and cigarette smoking.225 BV has not yet been categorized as an STI because of the absence of a recognized causative organism and lack of a counterpart in males. There is some evidence of BV-related bacteria found in men, but treatment of men has not affected BV in their partners.226 227 Despite the lack of STI status, sexual activity of one kind or another has usually been experienced by patients who have BV.228 229 For lesbian women, sexual transmission is common; there is a high occurrence of BV and concordance of flora in women who have sex with women.230 231 Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that do suggest sexual transmission of BV. One study showed that women in long-term, monogamous, lesbian relationships were also likely to have concordant normal vaginal organisms.232 When asked about triggers for their BV, women themselves identify a relationship with sexual activity (sex with a regular male or female partner, sex with a new partner, sex with an uncircumsized male, and sex without a condom).233 Condom use and estrogen based OCPs may be protective.234
There is an association between bacterial vaginosis and sexually transmitted infections.235 One study showed that women with herpes simplex type 2 infection had a 55 % higher risk of having BV than did those who were not infected.236 There is an increased risk of both gonorrhea and chlamydia in the presence of BV.237 238 There is a relationship between HIV and BV: women with BV may be more likely to contract HIV239 and women with HIV are more likely to have BV and for it to be persistent.240 241A study of HIV positive women in India showed an increased prevalence of BV, Candida, and Trichomonas in the study population, with higher numbers in those with lower CD4 counts.242
Clinical manifestations and symptoms
Approximately 50% of patients with BV are asymptomatic.243 Symptoms of BV include an unpleasant, “fishy-smelling” discharge often prominent after intercourse. The discharge is usually off-white, thin, and homogeneous. Dysuria and dyspareunia are rare, and pruritus, erythema, and inflammation are typically absent.244
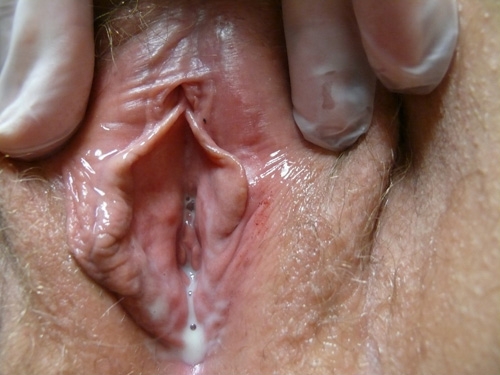
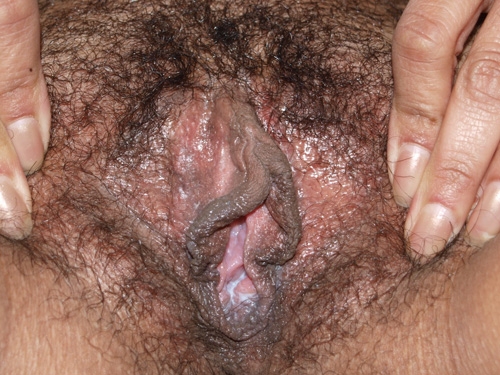
Diagnosis
History, speculum and pelvic examination, pH, whiff test, and wet mount are essentials of diagnosis. However, health care providers do not always follow these standards. One record review showed that whiff and pH measurements were performed in only 3% of women presenting with complaints. 42% of referring physicians did not perform microscopy, and 54% of visits resulting in medication showed inadequate evaluation of patients.245 246 Diagnosis is based on the presence of three of four of Amsel’s criteria (sensitivity >90%):247
Table P-10: Amsel’s criteria for diagnosis of bacterial vaginosis (three of four must be present)248
| · Homogenous, thin, gray-white discharge coating vaginal walls |
| · Vaginal pH greater than 4.5 |
| · Positive whiff test: the presence of fishy odor when 10% KOH is added to a sample of vaginal discharge. |
| · Presence of clue cells on wet mount. |
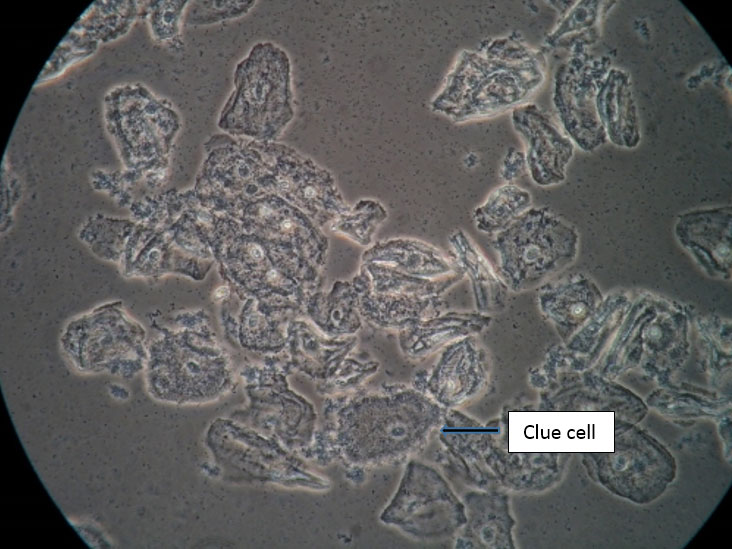
Clue cells are squamous epithelial cells covered with adherent bacteria, making the margins shaggy, uneven, and “moth-eaten” in appearance. These are the most reliable predictors in the diagnosis of BV.249 To be diagnostic, at least 20% of the epithelial cells on wet mount should be clue cells.
The Nugent test, (gram staining of vaginal secretions), is a reliable but clinically cumbersome method of diagnosis of BV (sensitivity 62-100%) usually utilized in a research setting.250 (See Table P-4: Nugent score table for gram stained vaginal smears for detecting bacterial vaginosis)
Vaginal culture has no role in diagnosis because there are no bacteria that are specific for BV. Although cultures for G. vaginalis are positive in almost all women with symptomatic infection, the organism is detected in up to 50 to 60 percent of healthy asymptomatic women; thus, its presence alone is not diagnostic of BV.251
PCR testing and DNA probes have become widely available for clinical use, but in a setting where microscopy is available, in-person evaluation is preferred to be able to detect the presence or absence of lactobacilli, WBCs, clue cells, pH and whiff.252
Five quantitative PCR assays are available: Max Vaginal Panel (Becton Dickinson), Aptima BV (Hologic), NuSwab VG (LabCorp), OneSwab BV Panel PCR with Lactobacillus Profiling by qPCR (Medical Diagnostic Laboratories), and SureSwab BV (Quest Diagnostics). Two of these assays are FDA cleared (BD Max Vaginal Panel and Aptima BV), and the other three are laboratory-based tests. Self sampling for BV and Candida has been found to be non-inferior to typical collection by a healthcare provider. This practice may alleviate the need for some follow up visits for patients with recurrent BV or to confirm the condition, but should not be relied upon.253
The following table, thanks to Dr Claire Danby, may help to clarify the choices of tests outside of pH, microscopy, and KOH and whiff testing:
Table P-11 Bacterial Vaginosis: Other diagnostics (from a 2021 lecture)
| Test | Method of testing | Sensitivity | Specificity | Notes |
| BD Affirm VPIII | DNA Probe | 95% 90.1% | 99% 67.6% | When combined with pH > 4.5 |
| OSOM BVBlue | Detects elevated sialidase enzyme | 88-94% | 91-98% | 3 studies, n = 57, 73, 288 |
| NuSwab (LabCorps) | PCR Based / NAAT for Atopobium vaginae, BVAB-2, and Megasphaera-1 | 96.7% | 92.2% | During validation trial |
| BD Max Vaginitis Panel | Real-time PCR for amplification of DNA targets, target spec. probes Lactobacilli spp., G. vaginalis, BVAB-2, A. vaginae, and Measphaera-1 | 90.5% | 85.7% | FDA-Cleared. The only one. |
| SureSwab (Quest) | DNA, Quantitative, Real-time PCR: GV, Lactobacillus, Mobiluncus curtisii and mulieris | Unsure | Unsure | Limited data |
Don’t use culture. Don’t rely on tests for G. vaginalis alone, as these tests lack specificity.
A Papanicolaou smear is not reliable for diagnosis of BV.254 If cytology suggests a shift in flora, women need standard diagnostic testing with examination, vaginal pH, whiff test and wet mount if symptomatic. Asymptomatic women do not need testing when a shift in flora is picked up on Pap smear.
Differential diagnosis
Other conditions that produce discharge and elevated vaginal pH >4.5 include trichomoniasis, vaginal atrophy, and desquamative inflammatory vaginitis (DIV). They can be distinguished by clinical and microscopy findings. Vaginal inflammation and dyspareunia are hallmarks of trichomoniasis, DIV, and atrophic vaginitis, not seen with BV. Increased parabasal cells are always seen with atrophy and DIV, and are not found with BV. Large numbers of polymorphonuclear leukocytes characterize trichomoniasis, DIV, and sometimes atrophic vaginitis but are not seen with BV. Motile trichomonads seen on microscopy are diagnostic of trichomoniasis.
Complications of bacterial vaginosis
BV is associated with a number of complications in both pregnant and non-pregnant women. It is causally associated with upper genital tract disease including endometritis, postpartum fever and post-hysterectomy cuff cellulitis, and post-abortion infection.255 256 BV is a risk factor for acquisition of STIs:257 HIV,258 HSV-2, gonorrhea, and Chlamydia.259 BV is often seen in women with pelvic inflammatory disease (PID), but its role as a risk factor is unclear.260 BV is associated with pregnancy-related complications of premature rupture of the membranes, premature delivery, and post-Cesarean wound infection.261 For this reason, treatment for all symptomatic pregnant patients is indicated. The exact nature of the association between BV and adverse pregnancy outcomes is unclear. The vaginal immune system may explain why only a minority of women with BV have adverse pregnancy outcomes. Although all women with BV lack classic signs of inflammation (vaginal erythema, vaginal leukocytes in wet mount), pregnant women with BV have silent evidence of inflammation: markedly elevated vaginal levels of the master pro-inflammatory cytokine IL-1b,262 strongly associated with elevation of another pro-inflammatory cytokine IL-8.263 Pregnant women who have BV and the presence of Mobiluncus were more likely, in one study, to have lower levels of both IL-1b and Il-8.264 It is hypothesized that the failure to increase IL-8, possibly caused by bacterial virulence factors such as a bacterial protease (sialidase) present with BV, may result in impairment of vaginal epithelial defenses, allowing easier migration of organisms to the upper genital tract.265 Treatment of high risk pregnant women with asymptomatic BV is not proven to improve outcomes.266
Treatment
The established benefits of therapy for BV in non-pregnant women are to relieve vaginal signs and symptoms and to reduce the risk for infectious complications after procedures such as abortion or hysterectomy. Other potential benefits might include a reduction in risk for other infections (e.g., HIV and other STIs). All women who have symptomatic disease should be treated.267 Trials that evaluated the use of anaerobic antimicrobial coverage (i.e., metronidazole) for routine operative prophylaxis before abortion and trials that evaluated this additional coverage for women undergoing hysterectomy demonstrated a substantial reduction in postoperative infectious complications.268 Therefore, treatment of both symptomatic and asymptomatic women who are to undergo pregnancy termination or hysterectomy is recommended. Because treatment of BV may also reduce the risk of acquiring other STIs, including HIV,269 some experts now support the concept of treating all women with BV regardless of presence or absence of symptoms. In the United States, treatment options have consisted of metronidazole or clindamycin, both available in oral and topical preparations. The Sexually Transmitted Infections Treatment Guidelines from the Centers for Disease Control and Prevention (CDC) include:
Table P-12: CDC Recommended treatment regimens for bacterial vaginosis in non-pregnant women270
| · Metronidazole 500 mg orally twice a day for 7 days, or |
| · Metronidazole gel, 0.75%, one full applicator (5 g) intravaginally, once a day for 5 days, or |
| · Clindamycin cream, 2%, one full applicator (5 g) intravaginally at bedtime for 7 days |
Table P-13: CDC recommended alternative regimens for bacterial vaginosis in non-pregnant women271
| · Clindamycin 300 mg orally twice a day for 7 days, or |
| · Clindamycin ovules 100 mg intravaginally once at bedtime for 3 days, or |
| · Secnidazole 2 gm packet of oral granules (sprinkled on applesauce or other soft food) in a single dose, or |
| · Tinidazole 2 gm orally daily for 2 days or 1 gm orally daily for 5 days |
*Note: Clindamycin ovules may cause breakdown in latex or rubber products (condoms or diaphragms)
Metronidazole therapy
The recommended metronidazole regimens are equally efficacious and metronidazole is the treatment of choice. Metronidazole gel is as effective as oral metronidazole, but the choice of agent is left to patient preference. One randomized trial evaluated the clinical equivalency of intravaginal metronidazole gel 0.75% once daily versus twice daily and demonstrated similar cure rates 1 month after therapy.272
Most comparative studies using multiple divided-dose oral regimens for one week achieved early rates of clinical cure in excess of 90%, and cure rates (by Amsel’s criteria) of approximately 80% at four weeks.273 There was a significantly higher short-term cure rate when the initial treatment with metronidazole therapy was extended from seven to 14 days.274 However, long-term cure rates (21 days after completion of therapy) were similar for both treatment regimens.
Side effects of metronidazole include nausea, diarrhea, headache, and metallic taste. The warning regarding Metronidazole and alcohol use (disulfiram-type reaction) is no longer applicable. Metronidazole has been associated with a range of CNS effects which include peripheral neuropathy, aseptic meningitis, ataxia, neurocerebellar toxicity, confusion or diorientation, dysarthria, encephalopathy, seizures, optic neuroathy, and vertigo, with most effects reversible within days to weeks of discontinuation; peripheral neuropathy symptoms may be prolonged.275
Clindamycin therapy
The standard topical vaginal therapy with 2% clindamycin cream (5 g of cream containing 100 mg of clindamycin phosphate) covers a seven-day regimen. Although it may be less effective than the metronidazole regimens,276 it is still recommended. Alternative dosing of 100 mg ovules, 1 intravaginally daily for three days, may be prescribed.
Limited data have been published that compare the clinical or microbiologic equivalencies of this regimen with other regimens. Cure rates do not differ between intravaginal clindamycin cream and ovules.277 278
The main side effect of clindamycin is diarrhea. Pseudomembranous colitis has been reported with both oral and topical clindamycin. Clindamycin cream is oil-based and might weaken latex condoms and diaphragms for 5 days after use.
Increased prevalence of clindamycin-resistant anaerobic bacteria in the vagina is seen post-treatment. 17% of bacterial isolates before therapy versus 53% of isolates after therapy have been reported with clindamycin.279 This effect persisted in most women for at least 90 days after clindamycin treatment. In contrast, increased resistance to metronidazole was not observed in women treated with that drug. The clinical significance of these findings is not yet clear. A study of Gram stain in women who had received metronidazole gel, single-dose clindamycin vaginal cream, or standard clindamycin cream, was performed at baseline and one month after treatment during three different clinical trials performed in a similar manner. In all three treatment groups lactobacilli increased to a similar degree.280
Allergy or intolerance to the recommended therapy
Intravaginal clindamycin cream is preferred in case of allergy or intolerance to metronidazole or tinidazole. Intravaginal metronidazole gel can be considered for patients who do not tolerate systemic metronidazole, but patients who are truly allergic to oral metronidazole should not be administered intravaginal metronidazole. Desensitization to metronidazole can be performed by consultation with an allergist, or as outlined in the literature.281
Tinidazole and secnidazole therapy
Tinidazole and secnidazole are second-generation nitroimidazoles. Compared to metronidazole, tinidazole has a longer half-life of 12 to 14 hours versus 6 to 7 hours and fewer side effects.282 It is at least as effective as metronidazole, as shown in randomized trials in the United States and elsewhere.283 A dose of 1 g orally once daily for five days has a slightly higher efficacy and fewer side effects than with shorter course therapy with more medication (tinidazole 2 g orally daily for two days).284 Secnidazole use for treatment of BV has the advantage of one-time dosing. Clinical cure rate of 2 g oral secnidazole was not different from oral metronidazole.285
Treatment during pregnancy
Treatment of symptomatic BV in pregnancy is recommended to relieve symptoms and possibly reduce risks. BV during pregnancy is associated with adverse pregnancy outcomes, including premature rupture of the membranes, preterm labor, preterm birth, intra-amniotic infection, and postpartum endometritis. Treatment may also reduce risk of STIs, including HIV. Although metronidazole crosses the placenta, there has been no evidence of teratogenicity or mutagenic effects in infants. Some experts avoid topical therapy in pregnant women because they believe oral treatment is more effective against potential subclinical upper genital tract infection, but intravaginal clindamycin and metronidazole gel have been shown to be safe in pregnancy. Tinidazole is not recommended for pregnancy.
Table P-14: CDC recommended treatment regimens for bacterial vaginosis in pregnancy287
Treatment of asymptomatic BV among pregnant women at low risk for preterm delivery has not been reported to reduce adverse outcomes of pregnancy. Therefore, routine screening of pregnant people without risk factors is not recommended.
Treatment of bacterial vaginosis in breastfeeding women
Infectious disease expert Jack Sobel recommends using oral Metronidazole 500 mg twice a day for seven days to treat lactating women with symptomatic BV.288 Serum levels received by infants are less than therapeutic infant doses would be and studies have shown no or minimal adverse effects in neonates exposed to this dosing. (Cohort studies have shown possible increased incidence of candidiasis or diarrhea).289 Oral clindamycin use (300 mg twice a day for seven days) is acceptable in breastfeeding women according to The American Academy of Pediatrics (AAP),290 however, the infant should be monitored for diarrhea, candidiasis (thrush, diaper rash) or, rarely, blood in the stool indicating possible antibiotic-associated colitis because of clindamycin’s potential to cause adverse effects on the breastfed infant’s gastrointestinal flora. Infant side effects are less likely with vaginal clindamycin than oral use since only about 30 percent of a vaginal dose is absorbed. Outcome data of maternal metronidazole use has not shown a significant increase in adverse events compared to use of other antimicrobials. Use of vaginal metronidazole has not been studied during breastfeeding. After vaginal administration, plasma levels are less than 2 percent of those after a 500 mg oral dose, so vaginal use of metronidazole during breastfeeding is unlikely to be of concern.291 Patients who are given tinidazome or secnidazole should avoid breastfeeding for 24-48 hours.
Treatment of recurrence and relapse in bacterial vaginosis
Recurrence of BV is common and troublesome for women. Cure for BV is reflected in normal pH and wet mount at four weeks after treatment. Since approximately 80% of patients successfully treated with therapy have a recurrence of symptoms within three months, the use of the word “cure” is misleading.292 There is little consensus on best practices to treat recurrent bacterial vaginosis (RBV). CDC Guidelines state that retreatment with oral or vaginal metronidazole or suppressive therapies using a variety of regimens is helpful, but the effectiveness diminishes once treatment is stopped.293 Recurrence may occur because lactobacilli are unable to reestablish themselves or because pathogenic bacteria are reintroduced or persist.294 More recent studies are focusing on the role of the biofilm in preventing clearing of BV with metronidazole.295 A recent retrospective cohort analysis shows promise: 105 women with RBV were treated with oral nitroimidazole (tinidazole or metronidazole) 500 mg twice a day for 7 days with simultaneous vaginal boric acid 600 mg for 30 days. (It is essential that boric acid never be taken orally because it is poisonous, and it must be kept away from children). Thereafter, they were prescribed twice weekly vaginal metronidazole gel for 5 months followed by a 6 month observation period. Initial treatment was successful in 92/93 patients. Maintenance gel prevented recurrence in 69.6% at 6 months. Long term cure at 12 months was seen in 69% of women. There was a signifiant loss to follow up at 12 months, but these findings certainly warrant further prospective study.296 The concomitant treatment of BV with disruption of the biofilm may be the key to longer symptom-free intervals or possible resolution. Although the anti-fungal effects of vaginal boric acid may prevent some overgrowth of yeast, many of the patients in this last study had to be treated with an anti-fungal, as well.297
Treatment of sexual partners
It is difficult to discern relapse from reinfection and to know what role partner colonization might play. It is logical to posit that BV infection is affected by flora of the penile skin. Male circumcision seems to decrease the prevalence of BV in female partners, but does not completely prevent it.298 Previous trials are limited due to many uncontrolled variables. Despite more than 60 years of research looking at the role of male treatment, there are not definitive data to support treatment of male partners.299 300 At the same time, there is good evidence that BV is related to sexual transmission in some way. Researchers continue to explore the question of partner treatment to improve outcomes.301 Despite BV being common amongst women having sex with women, to date, no female couples have been enrolled in treatment trials.302
Condom therapy
Reduced rates of recurrence have been documented when male sexual partners used condoms routinely with intercourse, or when the patient remained abstinent.303 Another report also suggests that condom therapy combined with medication is effective.304
Alternative treatments
Douching
Douching is not advised for treatment of BV. It is associated with an increase in BV secondary to the disruption of the normal vaginal flora.305
Boric acid vaginal acidifying treatment
Vaginal boric acid used alone or other agents that decrease the vaginal pH have not been shown to reduce the risk of BV. As noted above, there is promising evidence that, used in conjunction with long-term suppressive therapy with metronidazole, vaginal boric acid has the utility in the treatment of recurrent BV.
Probiotics
An exhaustive review of the literature by Joseph, et al, showed that there is no evidence, as of yet, to conclusively support using oral or intravaginal probiotics to decrease the incidence or recurrence of BV.306 One study using intravaginal Lactin-V (Lactobacillus crispatus CTV-05),307 more studies are needed. In addition, the use of dietary supplements containing live bacteria or yeast in immunocompromised patients has been shown to result in death in some patients.308
Vaginal microbiome transplantation
Vaginal microbiome transplantation has been studied in only a very limited number of patients and will need more study.309
Trichomonas vaginalis
Introduction
Although genitourinary trichomoniasis is the most common, non-viral, sexually transmitted infection in the world,310 it is not a reportable disease in the United States. Caused by the protozoan Trichomonas vaginalis, symptoms range from bothersome to non-existent. It accounts for 4 to 35 percent of vaginitis diagnosed in symptomatic women in the primary care setting.311 It is associated with serious sequelae: premature birth, low birth weight, and increases in human immunodeficiency virus transmission (HIV).312
Epidemiology
In 2016, the World Health Organization (WHO) estimated an incidence of 276 million new cases each year and prevalence of 187 million infected individuals.313 The prevalence of T. vaginalis in the United States is 2.1% among women ages 14-59, and 0.5% among men, based on a nationally representative sample of people who participated in the NHANES (National Health and Nutrition Examination Survey) in 2013-2016:
- Prevalence was 9.6% for African American women, 1.4% for Hispanic women, and 0.8% for non-Hispanic white women.
- For men and women, increasing poverty level, lower educational level, unmarried status, and having been born in the U.S. are associated with T. vaginalis infection.
- For women, younger age at first sex, greater number of sex partners, and a history of chlamydia infection in the past 12 months are associated with T. vaginalis infection.314 315
Etiology
Trichomoniasis is caused by a pear-shaped protozoan with flagellae, Trichomonas vaginalis, that may be located in the cervix and vagina, urethra, and paraurethral glands, as well as in the Bartholin and Skene glands. Unlike Chlamydia and gonorrhea, which require endocervical cells for optimal growth, T. vaginalis is primarily a vaginal pathogen. The entire vaginal surface is at risk, with implications for sampling, detection, and persistence of this protozoan.316 Once attached to the vaginal epithelial cell, T. vaginalis raises the vaginal pH and releases cytotoxic substances that cause epithelial breaks and inflammation.317 It is usually transmitted by sexual intercourse and is often associated with co-infection with other sexually transmitted diseases. It may be transmitted during penile-vaginal sex or through exchange of infected vaginal fluids or fomites among women who have sex with women.318 Although survival on fomites has been reported, fomites have no proven role in transmission of trichomonas.319 BV may be a risk factor for trichomoniasis, but the relationship between the two conditions is not well understood. Both vaginitides share the clinical signs of elevated pH and positive amine test. Many studies of BV used only a wet mount to exclude trichomonas, possibly resulting in under-estimation of trichomoniasis.
Clinical presentation
The vast majority of women have no symptoms at all: no itching, vaginal discharge, or odor.320 Asymptomatic carriage can exist for prolonged periods of time, making it impossible to ascertain the source of the infection. When symptoms develop, the typical presentation is a diffuse, malodorous vaginal discharge rather than itching. The classic green and frothy discharge is seen in about 10 % of culture proven infections.321 Punctate hemorrhages in the vaginal and cervical epithelium (strawberry spots) may be visible, but are present in only two percent of cases.322 Dysuria and frequency are present since the urethra is infected in the majority of women. Dyspareunia may also be present and post-coital bleeding may occur.
Complications
Trichomoniasis is often associated with other STIs, and may contribute to the development of pelvic inflammatory disease.323 It appears to increase the risk of herpes and human immunodeficiency virus 324 325 T. vaginalis has been found to double the risk of persistent human papilloma virus infection326 and increase the risk of cervical neoplasia.327 It is also a risk for post-hysterectomy cellulitis and tubal infertility. Trichomoniasis is associated with premature rupture of the membranes and premature delivery.328 The pregnancy-related complications, however, have not been reduced by treatment of asymptomatic infection.329
Diagnosis
As with Candida, bacterial vaginosis or any vaginitis, trichomoniasis cannot be diagnosed by history, signs, or symptoms alone. The elevated vaginal pH >4.5 and increased PMNs frequently noted are not diagnostic. Testing in females is more effective when vaginal secretions are used. With males, it is necessary to use urine samples. Males should be tested with culture, NAAT/PCR, or rapid tests designed for urine samples.
pH and wet mount
Identification of the motile trichomonads on wet mount has long been standard practice. If trichomonads are clearly identified on wet mount, no further testing is needed, although testing for other STIs should be offered.
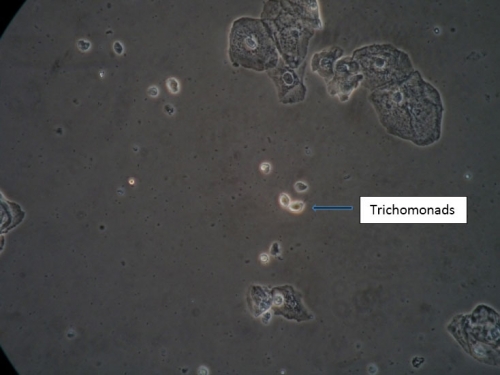
The wet mount must be performed within 10-20 minutes of obtaining the sample, or motility is lost. The poor sensitivity of wet mount (60-70%) and the time requirements of culture have hindered accurate diagnosis over the years. The CDC recommends adjunctive testing when T. vaginalis is suspected but not seen on wet mount.330
Other testing options
Culture
Before the development of NAATs (Nucleic Acid Amplification Tests), culture of vaginal secretions on Diamond’s medium, with its sensitivity of up to 95% and specificity of greater than 95%, was considered the gold standard for diagnosing trichomoniasis. However, the seven day wait for results and high cost now make culture desirable only in the case of pH>4.5 with increased WBCs on wet mount with no visible trichomonads, without availability of other testing.331 As the culture on Diamond’s medium was prone to contamination, another culture: InPouch system from BioMed Diagnostics was developed, using a two-chambered bag with the same efficacy as Diamond’s medium.332 However, molecular testing, with its aim of faster results, ease of use, and lower cost has now become the diagnostic tool of choice.333
Nucleic acid amplification tests/PCR
NAAT/PCR testing for T. vaginalis has high sensitivity and specificity and has become the gold standard now. The Aptima T.vaginalis assay from Hologic has a reported sensitivity and specificity range of 95-100% in all specimen categories (secretions and urine) and is FDA approved. When chlamydia and gonorrhea testing is desired, a separate Aptima assay is required, e.g. Aptima Combo 2. 334
Other tests have also been developed, not all FDA approved. The BD MAX vaginal panel, which is FDA approved, also tests for bacterial vaginosis and candidiasis. Amplicor (Roche) tests for T. vaginalis, N. gonorrhoeae, and C. trachomatis with a sensitivity of 88-97% and specificity of 98-99%. NuSwab VG (LabCorp) is a nucleic acid amplification single specimen assay for the detection of trichomoniasis, bacterial vaginosis, and vulvovaginal candidiasis.335
Rapid antigen and DNA hybridization probes
Rapid diagnostic kits using nucleic acid probes and monoclonal antibodies are changing the landscape of T. vaginalis testing. An unamplified RNA test (Affirm VP111; Becton Dickinson, Sparks, Maryland) is commercially available with a reported sensitivity of 80-90%, and a specificity of 99% for T. vaginalis compared with wet mount.336 It is also more sensitive than culture.337 Affirm requires 45 minutes to perform. The rapid antigen test (OSOM TV; Genzyme Diagnostics, Cambridge, Massachusetts), developed in 2003, is a point-of care, test-strip device that detects T. vaginalis membrane proteins in approximately ten minutes. The test has a sensitivity (83-90%) and specificity (98.8%) similar to culture.338 Both tests are performed on vaginal secretions.
Cervical cytology
The conventional Pap smear has a sensitivity of 51-63% and a common false positive rate of 7%,339 making it unacceptable for diagnosis because of its poor predictive value in a low prevalence population.340
Although liquid-based cytology has shown a low sensitivity (61), it has a high specificity (99%) and high predictive value (96.4%),341 leading some experts342 to believe that treatment of asymptomatic women with trichomonads noted on liquid based cytology is a reasonable approach. We feel that, especially in low-prevalence settings, a Pap smear by any technology giving a possibly false positive result for trichomonas, does not justify informing a patient that she has a sexually transmitted infection. Rather, the finding of possible trichomonads on Pap warrants confirmation by clinical evaluation with wet mount and culture, PCR testing, or rapid test.
Treatment
Treatment is indicated in all non-pregnant women diagnosed with trichomonas vaginitis, even if asymptomatic. Without treatment, up to one-third become symptomatic within six months. They continue to transmit the infection to sexual partners while untreated.343
For decades, effective treatment of trichomoniasis relied on the use of the 5-nitroimidazole drug, metronidazole. In 2006, the FDA approved another nitroimidazole, tinidazole for the treatment of T.vaginalis. The efficacy of the nitroimidazoles in curing 90-95% of trichomoniasis is well established, and most strains of T. vaginalis are highly susceptible to the drugs.344 Tinidazole is more expensive than metronidazole, reaches higher levels in serum and the genitourinary tract, has a longer half-life than metronidazole (12.5 hours versus 7.3 hours), and has fewer gastrointestinal side effects. Side effects to metronidazole include nausea, vomiting, headache, metallic taste, and dizziness. Intravaginal metronidazole gel does not reach therapeutic levels in the urethra and perivaginal glands. Because it is less efficacious than oral metronidazole, it is not recommended.345
Cure rates with medications other than the nitroimidazoles are so low that patients with allergy to metronidazole or tinidazole are best referred to an allergist for what is highly effective desensitization.346
Patients are no longer advised to avoid consuming alcohol during treatment with metronidazole or tinidazole because of the possibility of a disulfiram-like (Antabuse) effect.347
There are no studies on how long trichomonads remain viable after treatment is initiated or completed. Patients need education to avoid intercourse until they and their partners have completed treatment and are asymptomatic, about a week.
The CDC advises repeat testing of sexually active women in three months or at the time of their next visit.
Table P-15: CDC recommended regimens348for trichomoniasis in non-pregnant women
| Metronidazole 500 mg orally twice a day for 7 days, or |
| Tinidazole 2 g orally in a single dose (alternative treatment) |
Management of sexual partners
Sex partners of patients with T. vaginalis should be treated. Maximal cure rates in affected women are achieved when their male sexual partners are treated simultaneously.349 Data regarding female partners is not available, but sexual transmission of trichomonas between women is reported.350
Women should be instructed to avoid sex until they and their sex partners are cured (i.e., when therapy has been completed and patient and partner(s) are asymptomatic) or a follow up test at 3 months is negative.
Pregnancy
Symptomatic women in pregnancy should be tested and treated because trichomoniasis is associated with adverse pregnancy outcomes: premature rupture of membranes, preterm delivery, and delivery of small for gestational age babies. There is no standard at this time for routine screening in pregnancy.
Treatment of sexual partners of pregnant women is always indicated. In the untreated asymptomatic woman, re-infection of treated partners of untreated women can be minimized by abstinence or condom use.
Metronidazole 500 mg orally twice a day for seven days is the drug of choice for treatment of symptomatic trichomoniasis in pregnancy.351352
Over the years, clinicians have avoided use of metronidazole in the first trimester because the drug is mutagenic in bacteria and carcinogenic in mice. In humans, it crosses the placenta and has a theoretical potential for teratogenicity. Meta-analysis has not found any relationship between metronidazole exposure during the first trimester of pregnancy and birth defects.353 The Centers for Disease Control and Prevention no longer discourage the use of metronidazole in the first trimester. Vaginal metronidazole gel is not recommended because it does not adequately treat trichomoniasis. Tinidazole is not recommended in pregnancy.
Table P-16: CDC recommended regimen for symptomatic trichomoniasis in pregnant women
| Metronidazole 500 mg orally twice a day for seven days |
Breastfeeding
Use of metronidazole in breast-feeding mothers has caused concern because of the association with carcinogenesis in rodents. There are no human data to show an association between metronidazole and cancer,354 and maternal metronidazole use has not shown a significant increase in adverse events compared to use of other antimicrobials, although one study found a non-significant trend toward more loose stools in infants.355 With maternal oral therapy, breastfed infants receive metronidazole in doses that are lower than those used to treat infections among infants. If it is necessary to use oral tinidizole 2 g orally once, the patient should abstain from breastfeeding for 72 hours.356
Allergy to metronidazole
Desensitization to metronidazole can be performed by consultation with an allergist, or as outlined in the literature.357
Imidazole-resistant trichomonas vaginalis or treatment failure
If treatment failure occurs in a woman after completing a regimen of metronidazole 500 mg 2 times a day for 7 days and she has been reexposed to an untreated partner, a repeat course of the same regimen is recommended. If no re-exposure has occurred, she should be treated with metronidazole or tinidazole 2 g once daily for 7 days.358
The CDC will provide a special kit and guidance for performing drug-resistance testing if necessary. (https://www.cdc.gov/laboratory/specimen-submission/detail.html?CDCTestCode=CDC-10239).
Table P-17: CDC recommended treatment regimens359 in imidazole-resistant Trichomonas vaginalis
| Tinidazole orally in a single 2 g dose, or |
| Metronidazole at 500 mg orally twice daily for seven days (total dose 7 g), or |
| Tinidazole or metronidazole 2 g orally a day for five days (total dose 10 g) for failure of the other two doses. |
These regimens can be effective in patients with low levels of metronidazole resistance.
Therapeutic options include maximum tolerated doses of metronidazole, or preferably tinidazole (2 to 4 g daily in divided doses) for 14 days. Patients with high-level metronidazole resistance are usually successfully treated by prolonged and high-level tinidazole therapy, although cross-resistance to tinidazole is frequently reported.360 The optimal dose has not been established.
Other infectious causes of vulvovaginal symptoms
Streptococcal vaginitis
Group A streptococcus (Streptococcus pyogenes) {GAS} vaginitis in adult women is a rare condition limited to a few case reports.361 362 Vaginal colonization with GAS is low: in over six thousand vaginal and rectal swabs from women, there was a colonization rate of only a 0.03%.363
GAS is most often found in pre-pubertal girls; between 11% to 20% of vaginal swab samples obtained from girls with signs and symptoms of vulvovaginitis grew GAS in culture.364 Pediatric consultation is advised in this situation since vaginal streptococcal infection may suggest infection in other sites.
In the rarely reported cases in adult women, a family member, child, or adult was usually ill. GAS is possible in mothers of children with active GAS infection.365 GAS, found in the nasopharynx in patients with GAS pharyngitis, can spread GAS by droplets and physical contact. GAS is also possible in mothers of children who are GAS carriers. Although it is often unrecognized or ignored, carriage or exposure to a carrier is an important pathogenic factor in recurrent GAS infection. GAS can colonize the perineum, anus, vagina, and normal skin.366 367 Skin colonization is mostly noted in people with dermatological conditions such as psoriasis, eczema, and wounds. Gastrointestinal and peri-anal carriage may be evident in patients with pharyngitis even after pharyngeal infection has resolved and negative pharyngeal culture results have been obtained.368 There are two reported cases of women most likely infected by contaminated bed sheets and mattresses from their husbands: one with GAS necrotizing myofasciitis of the thigh, the other with rectal cultures positive for GAS. Air contamination can also result from carriers, regardless of the colonized site.369
Clinical features of GAS vaginitis include acute onset of frankly purulent discharge accompanied by itching, soreness and irritation, erythema, and labial edema. Microscopy of the discharge reveals a marked increase in polymorphonuclear leukocytes with cocci in chains. A Gram stain, if done, would also show chains of gram-positive cocci. Penicillin treatment after confirmation of Group A streptococcus by culture rapidly leads to cure. A standard treatment is Penicillin VK 500 mg four times daily for 10 to 14 days or clindamycin cream 2 percent vaginally for 7 to 10 days.
Table P-18: Treatment for culture-proven Group A Streptococcus vaginitis
| Penicillin VK 500 mg orally four times a day for 10 to 14 days or |
| Clindamycin cream 2% intravaginally for 7 to 10 days |
See document on Toxic Shock (LINK) for information on this condition. 370
See document on Methicillin resistant Staph aureus (LINK) for information on this condition.
Group B streptococcus
Group B streptococcus (GBS) commonly colonizes the vagina with rates up to 20% in pregnancy,371 and can lead to early and late onset neonatal sepsis and maternal postpartum endometritis. Whether GBS is a pathogen in the vagina is controversial. An increased prevalence of GBS is noted in 90% of women with desquamative inflammatory vaginitis (DIV),372 373 leading some clinicians to believe that GBS is the pathogen in this poorly understood vaginitis. Sustained improvement and/or cure following use of intravaginal hydrocortisone, however,374 support GBS as a bystander, not a pathogen in DIV. Randomized trials are needed. While a 14-day course of clindamycin 2% cream will often achieve remission, relapse after therapy is common.375 (DIV)
To date there is little to no medical evidence supporting GBS as a pathogen in vaginitis
Mobiluncus vaginosis
Metronidazole is effective against M. mulieris, but M. curtisii is relatively resistant.376 Horowitz reported M. curtisii overgrowth detected by phase contrast microscopy in patients previously treated with metronidazole. He treated with oral clindamycin 150-300 mg four times a day for 7 to 10 days.377 Other clinicians believe that the presence of Mobiluncus in significant numbers represents BV and treat according to CDC guidelines.
Foreign bodies
Malodorous vaginal discharge and erratic spotting or bleeding may represent inflammation and infection from a retained foreign body in the vagina, often a tampon. In most cases, removal of the foreign body is sufficient to clear the symptoms. Douching (never recommended) and antibiotics are unnecessary. Rarely, if symptoms persist more than 24 hours after the foreign body removal, vaginal clindamycin cream 2%, one applicator nightly for 7 days is indicated.
Condylomata
Extensive involvement of the vagina with warts can lead to discharge, bleeding, itching, irritation, and dyspareunia and will require eradication of the condylomata. (vaginal condyloma)
Unwelcome normal vaginal secretions
When a woman presents with the complaint of vaginal discharge, clarification regarding location is helpful for reassurance. Is the discharge from the vagina itself or is she concerned regarding secretions within the interlabial folds?
Although vaginal secretions can be evaluated by culture and other office tests, reassurance that secretions are normal requires information supplied by microscopy. There are no other reliable tests for the presence of parabasal cells or polymorphonuclear leukocytes (PMN) (the hallmarks of atrophy and inflammation), or for visualization of the vaginal flora that should have lactobacillus domination. With microscopy, one white cell per epithelial cell, normal, superficial, squamous cells, and a normal population of lactobacilli, the patient and clinician are reassured that the discharge is not bacterial. A yeast culture is essential at every evaluation because of the limited specificity of microscopy for Candida.
After a full evaluation has been performed without evidence of pathology, education can begin regarding normal vaginal secretions in a greater quantity than a woman wishes. We prefer the term “normal vaginal secretions” to physiological leukorrhea, since the latter has a pathological sound to it. We emphasize that the quantity of secretions varies from one woman to the next and that wetness (with the exception of incontinence) is part of femininity despite product marketing suggesting otherwise. We suggest avoidance of panty liners if possible, frequent changes of underwear, and a return in six to twelve months for reevaluation
In some cases, a woman with excessive “discharge” refers to the accumulation of normal, thick, yellow-white sebaceous secretions (smegma) in the interlabial folds. Bathing habits of women in the USA are not well studied; it is unknown if women routinely wash only the mons and labia majora, whether they expose the minora and interlabial folds, and/or include the vestibule. If the epithelium is intact and there are no signs of inflammation, she can be instructed to use fingertips to expose the area, warm water, and gentle soap (Dove bar) to remove the smegma. Demonstrating to her in a mirror the large numbers of normal sebaceous glands along the labial margins will educate her regarding normal vulvar secretion of lubricating sebum.
Cytolyic vaginosis
Cytolytic vaginosis (CV) was described in 1991 as a syndrome of pruritus, dyspareunia, vulvar dysuria, and cyclical increase in symptoms more pronounced during the luteal phase. Diagnostic criteria included the presence of discharge, pH between 3.5 and 4.5, absence of Trichomonas, Gardnerella, or Candida on wet smear, an increased number of lactobacilli, paucity of white blood cells, and evidence of cytolysis with disrupted epithelial cells and bare nuclei. Use of sodium bicarbonate douches was the recommended treatment.378
The existence of CV is controversial. Its non-specific symptom profile has made its diagnosis reliant on visual evidence of lactobacilli overgrowth and cytolysis on saline wet mount. Despite a call for more quantitative research in the original article, no changes have been made to the Cibley diagnostic criteria in the past 30 years, and wet mount microscopy remains the most frequently used method of diagnosis in the published literature
There have been only 10 published papers about CV. Some experts feel that CV is associated with Candida albicans,379 while others believe that CV is misdiagnosed as Candida albicans.380 Cytolysis, however, is a normal progesterone effect during pregnancy, the luteal phase of the menstrual cycle, and in perimenopause.381 In addition, the hydrogen peroxide formed by H202-generating bacteria can be auto-inhibitory;382 accumulation of excessive numbers of lactobacilli-generating toxic products is prevented by their own peroxide. Frequently, other reasons can be found for the symptoms that suggest CV: sometimes Candida, sometimes dermatitis or dermatosis with a normal vagina. Most experts do not recommend douching because of its lack of efficacy, disruption of vaginal flora, and association with ectopic pregnancy and upper genital tract infection. More importantly, there is currently no published evidence that sodium bicarbonate applied vaginally affects lactobacilli growth in the vagina or even raises vaginal pH, the supposed mechanism of action, for any extended period of time. Specifically, in addition to the lack of clinical outcome data, no studies have measured lactobacilli quantity before and after sodium bicarbonate treatment. 383
Contact/allergic reaction to douching, intravaginal treatment
Many women believe that the vulva and vagina are dirty. In addition, many think that the area should be dry and free of odor. Contemporary marketing practices for feminine hygiene products prey upon women in this regard.Women can experience contact irritation from these products. There are also cultural variations in hygienic practices. (LINK to Annotation J: Lifestyle issues).
Vulvovaginal atrophy (genitourinary syndrome of menopause, atrophic vaginitis)
Introduction
Both aging and the absence of circulating estrogen that occurs because of menopause can have profound effects on the female lower genital tract. Distressing symptoms may include sensations of vulvovaginal dryness, burning or irritation, dyspareunia, or urinary symptoms such as urgency, dysuria, or recurrent urinary tract infection (UTI). These symptoms have been referred to as vaginal atrophy or atrophic vaginitis. In 2014, a group of experts convened and recommended adopting the designation “genitourinary syndrome of menopause” (GSM) to reflect the collective symptomatic involvement of the vulva, the vagina, and the lower urinary tract.384 GSM is chronic and progressive for midlife and older women and is widespread. Women may experience none, some, or all of the symptoms, but for the diagnosis of GSM to apply, symptoms must be bothersome or cause distress and not be caused by another identifiable condition. (At the same time, more than one condition at a time can produce similar discomfort).
A decline in postmenopausal estrogen levels results in physiological changes that eventually affect virtually every organ system of the body.385 Sexual function and quality of life may be diminished significantly.386 Women may be reticent to disclose these symptoms to clinicians, whether from embarrassment, the expectation that with the natural aging process nothing can be done, or from fear of side effects of treatment. Clinicians may also be reluctant to intrude on the private lives of their patients by asking about GSM symptoms. In one study, only 32 of 100 postmenopausal women with chronic vulvovaginal symptoms had been prescribed topical or oral estrogen replacement. Many of these women did not fill their prescriptions.387 Although safe and effective treatments of vulvovaginal symptoms related to estrogen decline have existed for years, only 20-25% of symptomatic women seek help for symptoms.388 In addition, menopause is not the only reason for estrogen loss, (belying the label “genitourinary syndrome of menopause”). Premenopausal estrogen deficiency from a variety of causes, may go unrecognized. (See Etiology below).
Epidemiology
Between 27% to 84% of postmenopausal women will experience symptoms of GSM.389 390 391 392 393 394 Unlike other menopausal symptoms, such as hot flashes and sleep disturbances, that usually improve over time, vulvovaginal atrophy (VVA) is generally progressive in the absence of effective therapy. Vulvovaginal symptoms associated with estrogen deficiency can also occur during the female reproductive years. Premenopausal women can experience symptoms with the use of hormonal contraceptives, anticholinergic or chemotherapeutic medications, postpartum/breastfeeding, or inflammatory conditions. In a cohort of breast cancer survivors, vaginal dryness was present in 39.4% of the premenopausal patients.395
Pathophysiology
In the menopausal woman, an approximately 95% decline in estradiol levels396 leads to several gradual and persistent changes in the female genitals, where there is a high concentration of estrogen receptors. Estrogen maintains urogenital health through vascular, cellular, and structural mechanisms.
In GSM:
- The superficial vaginal epithelial layer thins, loses its elasticity, and can become friable
- Local blood supply decreases
- Glycogen and collagen content in the superficial epithelium wanes
- There is a change in vaginal microbiome as the glycogen that supports lactobacilli decreases
- This leads to an elevated vaginal pH
- Rugae flatten and disappear
- The vaginal canal shortens, narrows, becomes less distensible and can develop strictures
- As blood flow diminishes, secretions are lessened, and onset of lubrication during sexual stimulation is delayed.397 398
Etiology
Vulvovaginal atrophy can be caused by any condition or medication that results in a hypoestrogenic state:
- Natural menopause399
- Primary ovarian insufficiency
- Postpartum or breastfeeding
- Bilateral oophorectomy
- Hypothalamic amenorrhea and hypothalamic disease
- Use of anti-estrogenic medications: SERMS (although tamoxifen has estrogen agonist effects on the uterus and vagina),400 aromatase inhibitors, danazol, medroxyprogesterone acetate, gonadotropin releasing hormone agonists: leuprolide, nafarelin, goserelin
- Sustained use of some oral contraceptive pills (20 mcg of ethinylestradiol (EE)/100 mcg of levonorgestrel and 15 mcg of EE/60 mcg of gestodene)401
- Oral contraceptive pills with low levels of estrogen that ALSO contain drospirenone, desogestrel, or norgestimate. Testosterone contributes to lubrication in the vagina so birth control pills that lower a women’s testosterone levels (directly, by decreasing ovarian production or indirectly, by increasing SHBG) can contribute to vaginal dryness.
- Elevated prolactin with secondary reduction in ovarian secretion of estrogen
- Radiation, chemotherapy, or surgery that can lead to ovarian failure, including uterine artery embolization and uterine ablation
- Diminished androgen levels may contribute402
- Other factors that might promote atrophy of vaginal tissue, including tobacco use, cessation of coital activity, never having had a vaginal delivery, and vaginal surgery.403
Clinical manifestations
The symptoms of GSM may include sensations of vulvovaginal dryness, burning, vaginal discharge, dysuria, and dyspareunia, especially in sexually active women. While mild to moderate atrophy (GSM) may be asymptomatic or cause no discomfort for some women,404 it often worsens in the postmenopausal years. Symptoms advance from slight decrease in lubrication with sexual stimulation to marked dryness (75%), burning and/or pruritus, pain (15%), and dyspareunia (38%). Yellow-brown discharge and spotting, especially post-coitally, can occur when atrophy is severe. Irritative urinary tract symptoms such as frequency, urgency, dysuria, and hematuria are also part of GSM.405
It is important to remember that hormonal changes may not adequately explain postmenopausal dyspareunia, the predominant symptom attributed to the effect of low estrogen levels on female genitalia. Pain is also influenced by cognitive, affective, and dyadic factors. One study showed that, in comparison with hormone levels, cognitive-emotional variables (e.g., catastrophization, depression, anxiety) were found to be stronger predictors of pain than hormonal levels.406 On the other hand, the vaginal maturation index (VMI) assessment on microscopic wet mount showing a preponderance of parabasal cells and an absence of lactobacilli, associated with an elevated pH, is a significant predictor of both induced and intercourse-related vestibular pain.407
Diagnosis
History
An inquiry about symptoms of GSM in all peri and post menopausal women should be part of a routine review of systems. The EMPOWER survey of 2017 found that, in women who had never used any treatment, almost three quarters had never discussed their symptoms with a healthcare provider.408 As previously stated, the main reason for this reticence was an assumption that GSM was simply a natural part of aging and did not require remedy. Others were embarrassed to bring the topic up or did not think it was an appropriate topic to discuss with a health care provider. Women also lack basic information about GSM and possible remedies and would therefore benefit from routine queries and written handouts.409
Symptoms similar to atrophy (VVA associated with GSM) may be reported by postmenopausal women with other conditions: vulvovaginal infection, foreign body in the vagina, contact or allergic reaction, vulvar dermatoses, benign or malignant tumors, medical conditions such as diabetes, and psychological causes.410 In women of reproductive age, the clinician may not think of atrophy as a potential problem.
Annotation B (B: The patient’s history ) covers the details of taking a history for any vulvovaginal complaint. In the case of suspected GSM, the history should especially include partner relationship(s), current and past sexual activity, urinary symptoms, interventions tried, including home remedies, treatment goals (comfort, sexual activity), and level of distress with symptoms. If a woman has a history of cancer, details of site, hormone dependence, past and current cancer treatment, age at diagnosis and current age, type of menopause, etc, should be detailed. Discussion of radiation is included in Annotation O. Vaginal epithelium. Click radiation (LINK).
Physical examination
Signs of GSM observed during evaluation of the vulva and vagina vary with the degree of atrophy. With early stages, the epithelium of the vestibule is thin and dry. The vagina may appear mildly erythematous. With progression, there is diminished pubic hair and loss of the fat pad of the mons pubis. The labia majora become pendulous and the minora flatten and are less distinct. The prepuce may decrease in size, uncovering the clitoris in some women, and making it appear larger.411 As atrophic change progresses, the clitoris may recede and, in some cases, become completely flush with the surrounding tissue. As time goes on, vulvar and vestibular tissues appear thin, dry, and pale; the vestibule may be tender to touch, yielding a positive Q-tip test in a patient who does not have vulvodynia, but rather vulvovaginal atrophy (GSM).
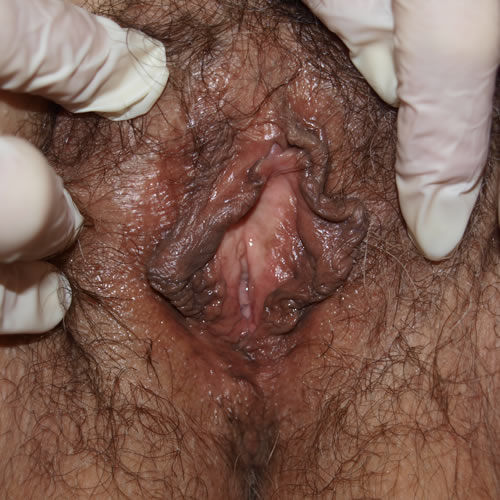
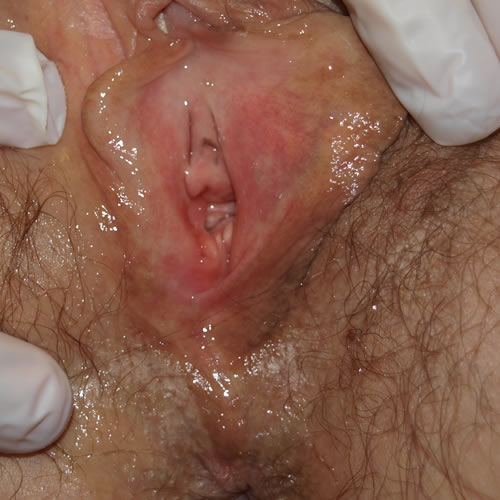
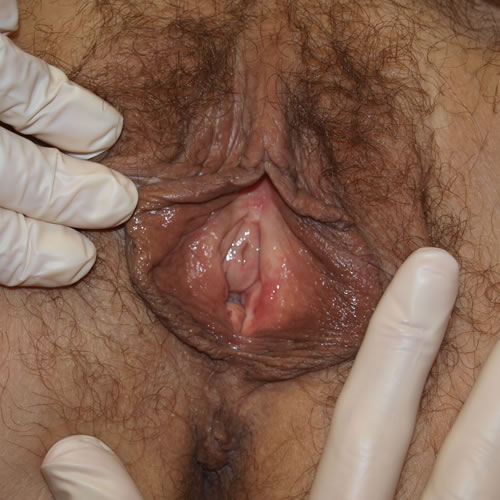
A urethral caruncle may be seen, manifesting, under estrogen deprivation, as a dark red, fleshy outgrowth representing mild prolapse of the distal urethral mucosa. These lesions may be painful and may be associated with dysuria or spotting. Caruncles are usually small, but can grow to 1-2 cm in diameter. (Atlas of Vulvar Disorders)
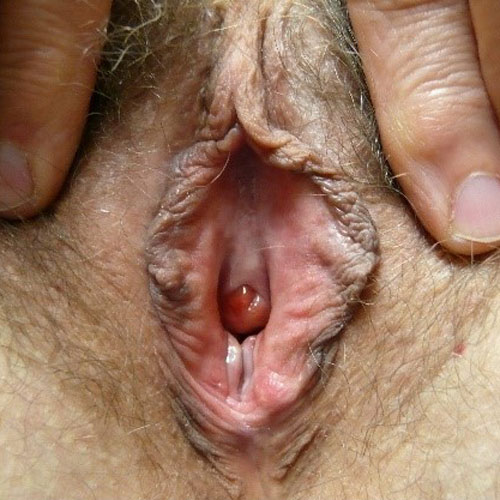
The severely atrophic vagina is poorly distensible, short, and narrow. The vaginal epithelium is smooth and dry, and may display color changes from erythema to pallor. It is easily injured by a speculum which may cause petechiae and bleeding. Some women develop a brown or yellow discharge. (Choosing an appropriate vaginal speculum is important for the patient’s comfort and to accomplish a successful exam. Use of a narrow Pederson or pediatric vaginal speculum with lubricant may be best. If well tolerated, the patient should inform the next clinician who sees her about what works for her. If not tolerated, the clinician may have to stop the exam). With advanced atrophy (GSM), the fornices may be obliterated, making the cervix flush with the vault. In some cases, use of even the thinnest Pederson or pediatric speculum may be impossible. (Application of 4% aqueous lidocaine for ten minutes prior to exam may be helpful). In these cases, it still may be possible to insert a Q-tip for pH, wet prep, and other testing.
A woman’s symptoms do not always correlate with physical findings. For example, a woman who is not sexually active may have few complaints, despite signs of advanced genitourinary atrophy on examination. Women who are not sexually active may, however, be bothered by other symptoms related to GSM, including vulvar discomfort with exercise or dysuria. In the absence of complaint, atrophic changes noted on exam do not necessarily require treatment.
Laboratory/point of care testing
Diagnosis of VVA/GSM is primarily a clinical one.
Natural menopause occurs with the final menstrual period, which is known with certainty only in retrospect one year or more after the event. An adequate independent biological marker for menopause does not exist, and there is no role for serial measurements of serum estradiol or follicle-stimulating hormone (FSH) levels for most women.
A single hormone value of low estrogen or elevated FSH may be misleading, since hormonal variation is characteristic of the perimenopausal years. In the late menopausal transition, fluctuations in serum concentrations of FSH and estradiol may be quite striking; high FSH and low estradiol values may be suggestive of menopause, but soon thereafter, one may see that FSH and estradiol have returned to the normal premenopausal range.412 Therefore, a single serum FSH value in the postmenopausal range, even with undetectable estradiol (and inhibin) levels does not provide reliable evidence that menopause has occurred.413
The vaginal maturation index (VMI), described in a study by Meisels in 1966414 has been very helpful in accurately diagnosing vaginal atrophy as well as ruling out other conditions that may mimic it. His studies defined estrogenization norms and showed that a cytologic measure of vaginal epithelial maturity reflected the effect of circulating estrogens, androgens, and progestogens.415 Lindau, et al, replicated Meisels’ work in 2017. Interestingly, while the norms established by Meisels held true proportionately, vaginal estrogenization was higher in every age group than reported in the 1960’s study and previous hormone therapy use, obesity, and African American race were each independently associated with a higher MVI. Lindau’s group points out that rates of obesity in US women have increased from 15.0% in 1960 to 35% in 2012,416 417 and they theorized that increased exposure to environmental estrogens might also account for increased maturation values in contemporary women. Lindau’s study confirmed that low maturation value was associated with vaginal dryness during sexual vaginal penetration,418 however, they found that, in their cohorts, “there was not an age-related decline in vaginal estrogenization after menopause and that post meopause, half of all women will exhibit vaginal atrophy, but half will not.”
Hypoestrogenism induces a decline in the vaginal epithelium glycogen level, which is the substrate for lactobacilli. The consequent reduction of lactobacilli leads to an increase in the pH and a shift in not only the proportion of superficial, intermediate and parabasal cells, but a possible shift in the resident microbiota. The decrease in lactobacilli and increase in bacterial diversity, including more enteric bacteria, can result in a brown or yellow, and sometimes malodorous vaginal discharge.
Vaginal pH testing and microscopy, essential to determine the VMI and to diagnose vaginal infections or imbalances in microbiome, are invaluable aids not only to diagnosis of genitourinary atrophy but also to evaluation of treatment. Vaginal pH may be as high a 7.0 in postmenopausal women not on estrogen therapy, but will revert to lower levels (4.5-5.0) in women using vaginal estrogen.419
The wet mount may show greater than one white blood cell per epithelial cell, and will show reduction in mature squamous epithelial cells, the presence of immature parabasal cells, and reduction or loss of lactobacilli. The pH and microscopy synopsis illustration in Microscopy pH and Microscopy Synopsis Illustration shows that changes in the post menopausal wet mount are normal but also support the patient’s atrophy-related complaints.
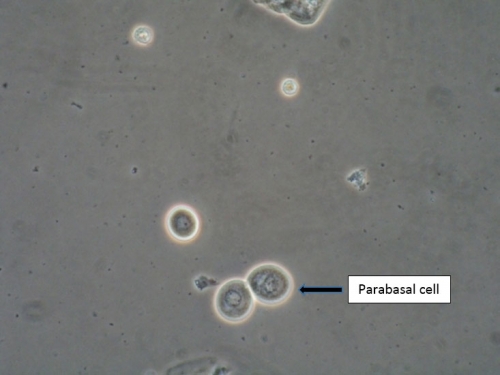
Overgrowth of other flora, usually bacterial, may or may not be present. (Candidiasis and bacterial vaginosis are rare in postmenopausal women not using estrogen).420 The wet mount of atrophy (GSM) is sometimes identical to that of desquamative inflammatory vaginitis (DIV) or vaginal lichen planus, although the wet prep in the case of GSM tends to be less cellular than that of DIV, usually showing few WBC’s and a preponderance of parabasal cells. Please see Box #5 in the Microscopy Table of conditions associated with an elevated pH to understand the process of diagnosis for VVA versus DIV. Elevated pH Microscopy Table: Box 5 illustrations A trial of adequate local estrogen is essential to differentiate these conditions in cases where there is a reason for low estrogen.421
Possible adjunctive point of care testing includes the Vaginal Health Index (VHI) in which only a vaginal pH is done and is included in a scoring system that clinically measures elasticity of tissue, fluid volume (pooling or secretions), epithelial integrity, and moisture (coating).422
Table P-19 Vaginal Health Index 423
| Score | 1 | 2 | 3 | 4 | 5 |
| Elasticity | None | Poor | Fair | Good | Excellent |
| Fluid volume (pooling and secretions | None | Scant amount, vault not entirely covered | Superficial amount, vault entirely covered | Moderate amount of dryness (small areas of dryness on cotton-tip applicator) | Normal amount (fully saturates cotton-tip applicator) |
| pH | > 6.1 | 5.6-6.0 | 5.1-5.5 | 4.7-5.0 | <4.6 |
| Epithelial integrity | Petechiae noted before contact | Bleeds with light contact | Bleeds with scraping | Not friable, thin epithelium | Normal |
| Moisture (coating) | None, surface inflamed | None, surface not inflamed | Minimal | Moderate | Normal |
This assessment system, used originally for research, can be helpful in conceptualizing the signs of atrophy the clinican should look for.
A 2022 study attempted to find a tool for direct and objective measurement of vaginal moisture. Using a calibrated filter paper similar to the opthalmic Schirmer test for eye moisture, a “modified Schirmer test” was found to be valuable in assessing the degree of vaginal dryness in study and control patients and correlated well with vaginal pH of patients with atrophy, the VHI score and the Visual Analogue Score (VAS) of dryness during intercourse.424 The use of pH and microscopy, which identifies pathogens and imbalances in the microbiome as well as revealing the vaginal maturation index (VMI), is essential in diagnosing all vulvovaginal disorders and continues to be the gold standard in the diagnosis of vaginal atrophy.425 Clues from the physical exam and from the pH, KOH and wet mount, on both first assessment and follow up visits, guide the need for further testing.
Pap smears and vaginal atrophy
Nuclear enlargement seen in atrophic epithelial cells represents one of the criteria for atypical squamous cells (ASC) and low grade squamous epithelial lesions (LGSIL), leading to an abnormal Pap smear.426 For this reason, a Pap with LGSIL can be followed in a woman who is negative for high risk HPV. Adequate local estrogen may be recommended prior to cytologic follow-up.
Differential diagnosis
GSM in the postmenopausal woman may accompany and worsen any vulvovaginal complaint. It is common, for example, to see a postmenopausal woman with symptoms of lichen sclerosus and low estrogen, requiring treatment for both. Symptoms from an over-the counter irritant such as witch hazel, benzocaine topical anesthetics, or aloe that she has applied for relief may be contributing factors to the clinical picture.
Vulvovaginal atrophy (VVA) of GSM may masquerade as:
- Vulvovaginal infection (See sections on Candida, BV, Trichomonas, DIV)
- Foreign body in the vagina
- Contact or allergic reaction
- Vulvar dermatoses: lichen sclerosus, lichen planus (Atlas of Vulvar Disorders)
- Vaginal lichen planus (Annotation N and O)
- Benign or malignant tumors. Cervical, vaginal, and upper tract cancers must be ruled out if vaginal bleeding is a complaint.
- Medical conditions such as diabetes
- Psychological causes
- Vulvodynia
- Any condition causing troublesome vulvovaginal symptoms.
Treatment
Treatment of GSM is aimed at alleviation of symptoms by reversing atrophic vulvovaginal changes. For the woman with GSM, after excluding other causes of her symptoms, treatment can be approached systematically. Symptom reduction may take 1 to 3 months, and continued therapy is generally required because symptoms are likely to recur on cessation of treatment. It is important to emphasize this point with patients, who often expect a “quick fix” and may discontinue therapy once tissue integrity is restored. Data on symptom recurrence, however, is lacking.
Although women receiving systemic hormone therapy are less likely to experience VVA and sexual complaints,427 systemic therapy may be inadequate for vulvovaginal comfort in some patients.428 A variety of treatment modalities exists. These include ongoing sexual activity, lubricants, moisturizers, vaginal estrogens, vaginal androgen, pelvic floor physical therapy, vaginal dilators, vaginal laser, topical lidocaine, and systemic androgens, SERMS, and estrogen. Herbal products appear to be ineffective for the treatment of GSM.429
Non-hormonal therapies
Although less effective than hormonal treatments, some women and physicians prefer non-hormonal therapies (moisturizers and lubricants) as the first approach to alleviate vulvovaginal symptoms related to menopause. Moisturizers and lubricants are over-the-counter products with FDA approval as “cosmetics.” They may aid with symptoms but do not reverse atrophic vaginal changes.
Lubricants
Lubricants are temporary measures that may relieve vaginal dryness and minimize friction and irritation for both partners’ genitals during foreplay or penetrative sex. Lubricants have a short duration of action and therefore must be applied frequently. Lubricants can be in liquid or gel form: water-based, oil-based, or silicone-based. In general, water-soluble lubricants are associated with fewer genital side effects than silicone lubricants.430 431 Women with vulvovaginal problems should avoid lubricants that contain perfumes, flavors, or warming ingredients.
Decrease in lubrication in postmenopausal women is directly tied to a reduction in systemic and local estrogen. Decreased lubrication in the premenopausal woman can arise from breastfeeding and medications including antihistamines, OCPs, progesten only pills, decongestants, anticholinergics/antimuscarinics, GnRH agonists, SSRIs, chemotherapy, etc. Emotional stressors (job, money, relationships) can also interfere with arousal and lubrications. Older women tend to use lubricants to alleviate the discomfort of VVA, whereas younger women tend to use lubricants to enhance sexual experience.432
Table P-20: Lubricants: personal preference433
| WATER BASED | OIL BASED | SILICONE BASED |
| Thickening agents in a water-soluble base differ by: consistency, feel, duration of action | Oil: mineral, olive, almond, coconut, other | Silicon Dimethicone |
| Dry quickly; reapplication necessary. Osmolality determines irritation | Slippery
Messy/greasy |
Slippery; “coating” effect |
| Compatible with latex condoms | Break condoms, diaphragms | Not compatible with silicone rubber sex toys |
| Sticky | Possible odor | Bad taste |
| May contain preservatives: parabens, propylene glycol or antiseptics: chlorhexidine-glycerin | Non-irritating | Non-irritating |
| Wash away easily | Water-resistant, difficult to wash off body; stain clothes or sheets. | Water-resistant, difficult to wash off body, clothes or sheets. Need soap for removal. |
| Astroglide Free, Slippery stuff, Just Like Me, KY Jelly, Pre-seed, Liquid Silk, Good Clean Love, YES personal lubricant | Elegance Women’s lubricants
Mineral oil, olive oil, almond oil, coconut oil, corn or canola oil |
Uberlube, Astroglide X, Pjur, Eros, Pink ID Millenium, Wet Platinum, KY Intrigue |
Water based lubricants are often preferred for their ease of use, but experimentation may be necessary to avoid irritation. Preference should be given to lubricants that have a pH and osmolality similar to that of the vagina. The higher the osmolality of the lubricant, the higher the potential to cause irritation of the mucosa and tissue damage.434 The WHO recommends using a lubricant with a pH of 4.5 (women) and an osmolality below 1200 mOsm/kg.435 436
Table P-21: Osmolality of water based lubricants
| OSMOLALITY | MUCOSAL INFLUENCE | PRODUCTS |
| Iso-osmotic 314 mOsm/kg | No irritation | Pre-seed |
| Hypo-osmotic 13-32 mOsm/kg | Negative mucus production | FemGlide, Slippery Stuff |
| Hyper-osmotic 1491-5848 mOsm/kg | Mild to severe irritation | Replens, KY jelly, Astroglide |
Lubricants and fertility
Lubricants may affect sperm integrity and function, thereby decreasing fertilization potential. Motility is significantly decreased by saliva, olive oil, and KY jelly but not by baby oil.437 In a recent study, Markham, et al concluded that with the exception of egg white, which appeared to be a safe option for couples trying to conceive, all lubricants caused significant (P<0.001) reductions in sperm forward progression compared with untreated controls.438 Comparisons of the commercially available lubricants revealed statistically significant differences in forward progression motility: Pre-Seed was superior to Optilube (P<0.001) and to Yes Baby (P<0.001) at 2-4 hours after exposure.439
An earlier study showed that motility was significantly decreased by FemGlide, Replens, and Astroglide, but not by Pre-Seed.440 Sperm chromatin integrity is significantly decreased by KY-jelly and FemGlide, but not by Pre-Seed. 441
Table P-22: pH and osmolality of lubricants and sperm 442
Lubricants and condoms
It is noteworthy that oil-based lubricants can erode condoms; however, most brands of water-based and silicone-based lubricants are latex-safe and condom compatible. For example, the silicon lubricant, Uberlube, is compatible with natural rubber latex and polyisoprene condoms. Uberlube is not compatible with polyurethane condoms. Patients should not use products made with oil (cooking, baby, coconut, mineral, etc) as lubricants for latex condoms. The oil weakens the material the condom is made from and can cause the condom to break. Polyisoprene in non-latex condoms reacts to oil-based lubricants in the same way latex does. Polyurethane condoms, however, are safe to use with oil-based lubes.
Lubricants and parabens
Parabens are common preservatives that have been used in the cosmetic and food industry and are found in some lubricants.443 They are known to have anti-bacterial and anti-fungal properties. Any product with ‘paraben’ as a suffix contains parabens. KY jelly and Replens contain methylparaben (a type of paraben) and Astroglide contains methyl and propyl paraben plus propylene glycol.
Personal-care products are significant contributors to paraben exposure. Berger, et al. found that adolescent girls who wear make-up daily had a 20-fold increase in propylparaben in their urine compared to those who rarely wear make-up.444 This suggests that parabens are absorbed systemically from topical use. Parabens are weakly estrogenic and there is concern that they present a risk, as they have been found in human breast cancer tumors.445 Parabens are also thought to affect the mechanism of cell turnover, making an association with carcinogenesis theoretically possible.446
Lubricants and glycerin
Glycerin is widely used in medical, pharmaceutical, and personal care products, including lubricants. Typically added to promote lubrication, glycerin is used to reduce the loss of moisture. In some patients it may cause dryness. Glycerin is a metabolic byproduct of sugar so it may promote yeast infections.447 Glycerin is also thought to damage the flagellar membrane in sperm and thus to be the potential causative agent in decreasing sperm motility.
Organic lubricants
Organic approaches to lubrication include products free of petrochemicals, parabens, and fragrance. The organic ingredients are infused in some cases with flowers and herbs. Water based formulas include aloe vera, agar agar and xantham gum. Examples included Emerita® and Me Again®. It is important to remember that natural substances such as aloe and hyaluronic acid can still act as irritants.
Lubricant recommendations
Based on informaton about pH, osmolality, ingredients, effects on condoms and diaphragms, and sperm influences, our recommendation for a lubricant for premenopausal patients with vaginal dryness who are attempting pregnancy is Pre-Seed.
As for postmenopausal patients, these are personal products and trial and error is sometimes necessary. The authors have received positive feedback from colleagues and patients about the use of Uberlube. Uberlube is pure silicone with a trace amount of vitamin E. Uberlube uses no animal products, is vegan friendly, has no glycerin, no parabens, no scents, and no flavors. It is anhydrous, so osmolality has not been studied. Uberlube, is compatible with natural rubber latex and polyisoprene condoms. Uberlube is not compatible with polyurethane condoms.
Moisturizers
Another non-hormonal approach to some aspects of GSM is the use of vaginal moisturizers. Without sustaining levels of estrogen, vaginal fluid secretion decreases from 3-4 g/4 hours to 1.7 g/4 hours.448 This decrease results in tissue drying. Although topical estrogen in the form of creams, tablets, time-release vaginal rings, etc, restores the vaginal maturation index to the pre-menopausal picture and relieves dryness and dyspareunia, many women do not want to use hormones or have been advised not to.
Unlike lubricants, whose actions are short lived and are best used in concert with sexual activity, vaginal moisturizers are longer lasting and act as an aid to day-to-day comfort, irrespective of the timing of sexual activity. Moisturizers are designed to adhere to the vaginal mucosa, thereby promoting rehydration and mimicking normal lubrication.449 These products maintain tissue integrity, elasticity, and pliability and should be used regularly (daily to every three days). The goal of use is to reduce daily symptoms of GSM as well as to decrease dyspareunia.
Moisturizers contain water as well as emollients and preservatives. Additional additives may include perfumes, flavors, spermicides, dyes, and warming agents, any of which can be irritating to aging vulvovaginal tissue. Some moisturizers contain propylene glycol, aloe (natural, but still sensitizing to some), or chlorhexidine–all known irritants. Patients may need to try different formulations to identify the product that is best for them.
A well-known moisturizer is Replens®, a bioadhesive polymer containing purified water, glycerin, mineral oil, polycarbophil, carbopol 974P, hydrogenated palm oil, glyceride, and sorbic acid. It binds to the vaginal epithelium, releasing purified water to form a moist film over the vaginal tissue. For most women, moisturization is maintained if the product is used on a regular schedule of every two to three days; Replens® does not need to be applied just before intercourse. With ongoing use, some women note the build-up of polymer residue as white, cotton wool-like material in the vagina. Replens was originally reported to reverse atrophy (GSM), but these results came into question when it was reported that it caused no change in the maturation index or nuclear area. 450
Other vaginal moisturizers include RepHresh, Feminease, Luvena, Silken Secret, KY SILK E, and Me Again. An internet search for vaginal moisturizers turns up many additional products, including products infused with CBD.451
A new class of moisturizers utilizing hyaluronic acid are now available including Revaree and Hyalo GYN. Hyaluronic acid is a natural polysaccharide in the skin, connective tissue, and eyes, and works by binding to water to retain moisture. It is added to many commercial skin care and wound-healing products because of its purported effect of drawing moisture to wherever it is applied. There are several small head-to-head trials that show products with hyaluronic acid provide comparable efficacy to estrogen-based treatments with vaginal dryness and dysparunia as the primary end points.452 A recent prospective, randomized study compared application of intravaginal 1% estradiol cream with intravaginal Vagisan, a hormone-free oil-in-water emulsion (cream), certified as class IIb medical device for intra- and extra-vaginal use (CE 0483) with a lipid content of 23%, a pH adjusted to 4.5 with lactic acid and an osmolality of 374 mOsm/kg, which is in accordance with the WHO’s recommendation of < 380 mOsm/kg for personal lubricants.453 Results showed that the non-hormonal cream was “not inferior” to the estradiol cream and complaints of side effects were about the same in both groups.
There are few published reports on the safety, efficacy, and irritation potential of vaginal lubricants and moisturizers. Women with concerns can test products on a small patch of skin for 24 hours before using them intravaginally. If the product they test successfully on the skin still causes irritation in the vagina, a woman can switch to an iso-osmolar, propylene glycol-free or silicone-based product. Cost of these products p-20varies considerably and will be a consideration for some users.
Table P-23: Vaginal moisturizers
Local vaginal estrogen treatment
While effective for many menopausal complaints, systemic estrogen, even at higher doses, fails to relieve vulvovaginal symptoms in 10% to 25% of women.454 Some oral estrogens (Premarin, Estrace) are FDA approved for the treatment of VVA. Among transdermal estrogen creams and gels, only Estrogel 0.06% is approved for the indication.
Vaginal atrophy (GSM) is effectively treated with local estrogen, either intravaginally or topically at the introitus. Formulations include vaginal creams, a vaginal ring with time-released medication, or vaginal tablets. Much lower doses of estrogen than those used systemically, applied directly to the vagina, result in improvement in GSM with minimal elevation of serum estradiol levels.455 Local vaginal therapy is therefore preferred over systemic estrogen for this indication.456
There are multiple vaginal estrogen products. A Cochrane review of trials on over two thousand women concluded that conjugated estrogen vaginal cream, estradiol vaginal tablets or the estradiol vaginal ring were all equally effective for relief of symptoms.457 Symptom relief can be expected within three to four weeks of therapy but may occur sooner.458 Improvement in the vaginal maturation index (VMI) has been reported two to four weeks after the initiation of vaginal conjugated equine estrogens (Premarin) or estradiol vaginal tablets (Vagifem).459 Vaginal pH falls to its lowest levels by the third week of vaginal estrogen treatment. Superficial cells continue to increase during a 12-week course of therapy.460 Governmental regulatory bodies agree that data support the efficacy of vaginal estrogen therapy for vaginal atrophy (GSM).461
Adverse events associated with use of vaginal estrogen therapy include vaginal discharge, vulvovaginal candidiasis, vaginal bleeding, and breast pain. Long-term follow-up studies on endometrial safety are not available although expert concern is low. Mild transient candidiasis is common, sometimes not requiring treatment.462 Use of vaginal estrogen has not been found to be associated with risk of breast cancer. Vaginal estrogen treatment in women not exposed to systemic menopause hormone therapy for greater than 18 years showed the risk of cardiovascular disease, cancer, and hip fracture to be similar to that of non-users of vaginal estrogen.463
Choosing which form of vaginal estrogen to use must take into account patient preference, concern about estrogen exposure, and cost. Dose and length of treatment need to be individualized. For women with significant vulvar symptoms, including cracking and fissuring, initial treatment with externally applied creams in addition to internal application should be considered. The patient should be counseled that response to therapy usually takes from 4 to 6 weeks. Once symptom relief is obtained, treatment should continue indefinitely. To date, clinical studies have not extended beyond a year in follow-up, and no time limits for therapy use have been established.
Local vaginal estrogen is so effective for treatment of vaginal atrophy that “atrophy unresponsive to estrogen” should prompt re-evaluation for other sources of complaints that continue after an appropriate duration of local estrogen treatment: e.g. dermatosis such as lichen sclerosus or lichen planus, contact dermatitis, other vaginitis, especially desquamative inflammatory vaginitis, and vulvar pain of all types.
Although circulating estrogen concentrations generally remain within the menopause range with low-dose vaginal estrogen therapy, the package insert for these products includes the same black boxed warning regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and probable dementia that accompanies systemic estrogen products. Patients must be informed about the differences between low-dose vaginal and systemic estrogen therapy and be prepared for the boxed warning, or else they may not initiate prescribed treatment.464 Multiple studies have shown that topical estrogen products do not carry the same risk as oral medications.465
As mentioned above, cost is a consideration and insurances vary in their preferred products. Savings coupons for some products are available but do not work with federally funded health plans (Medicaid, Medicare). Many estrogen products now require “prior authorizations:” applications by office staff to insurance companies to explain why a particular product is needed. This work adds to costs. https://en.wikipedia.org/wiki/Prior_authorization
Vaginal estrogen creams
Vaginal creams contain conjugated equine estrogen (Premarin cream), estradiol (Estrace cream or generics), or promestriene. It is possible to compound a cream with the weaker estrogen, estriol. Although commercial estriol is available in Europe, it is not FDA approved in the USA.
Conjugated equine estrogens (CEE) (Premarin) cream 0.625 mg/gm is effective in doses of 0.5 to 2 grams (0.3 mg to 1.25 mg CEE) delivered in schedules ranging from once daily to two or three times weekly.466 The recommended initiation of therapy is 0.5 g of CEE daily into the vagina for three weeks followed by twice-weekly administration thereafter. This amount comprises one-eighth of the applicator sold with the cream and contains 0.3 mg of conjugated estrogen. Vaginal cytology in postmenopausal women given daily 0.5 g conjugated estrogen intravaginally, changed to cytology of normal premenopausal women, even though serum estrone and estradiol concentrations did not increase substantially.467 There is no similar information for estradiol vaginal cream.
Estradiol cream, obtained from soy and yams, can also be given by vaginal applicator at a dose of one-eighth of an applicator or 0.5 g (which contains 50 mcg of estradiol). The package insert indicates a maximal dose of 2.0 g daily, but this is not generally necessary. This produces premenopausal plasma levels of estradiol468 and should not be used long-term without endometrial assessment.
Many women object to the use of equine estrogen and request estradiol; some insurances, however, will not cover estradiol but do cover equine estrogens. The patient may need to inquire about her coverage prior to initiation of therapy.
Estriol is a relatively weak natural estrogen that is used outside the US to treat vulvovaginal atrophy. Brand names include Ovestin and Gynest. A low-dose estriol vaginal gel (0.005%) has been shown to signifiantly improve the vaginal maturation index (MVI) and vaginal pH compared with a baseline evaluation in postmenopausal women.469 Estriol is not approved by the FDA for any indication.
Occasionally, topical estrogen cream can burn slightly for a minute or two after application, even in women with normally estrogenized tissue. In the presence of severe VVA, the loss of the normal epithelial barrier may cause burning with any contact, even plain water in extreme cases. Estrace and Premarin cream may burn for this reason, or because they contain preservatives and alcohols that irritate. This situation can sometimes be avoided by restoring the epithelial barrier: the patient is asked to soak twice daily (to hydrate the epithelium) for five minutes in warm water, then apply a film of plain Vaseline around the introitus (to seal in the moisture and restore the epithelial barrier). After two weeks, she should try a small amount of topical estrogen as a test. If that is tolerated, she may proceed with normal application of the product. An alternative is to compound estradiol 0.1% in petrolatum or another bland base, e.g. versabase or lipoderm base. Compounding half-strength estradiol 0.05% cream is yet another option.
If the above measures are not successful, it is important to realize that the physical application of the topical treatment may be acting as a trigger to provoke pain. Encourage the woman to use gentle, light, and slow touch to expose the tissues and to apply the medication.
Estrogen creams applied topically may NOT be irritating, on the other hand, and may offer more immediate soothing relief of symptoms, possibly due to the emollient effect. Topical digital application in the vestibule directs the cream precisely and effectively, while internal use with an applicator helps improve the vaginal environment. Some women, however, consider the creams messy. (Vaginally applied creams do drip out in the form of discharge during the day after night time application). Sensitivity to the vehicle used in the creams has also been reported. With estrogen cream, the patient has the responsibility of preparing the dose because the amount of cream inserted is not in a prepackaged dosing unit. This has the potential for error, with inadvertent higher-than-recommended dosing. The potential risk for male partner estrogen absorption remains unknown.
Vaginal estrogen tablets
Vaginal estrogen tablets contain 10.3 mcg of estradiol hemihydrate which is equivalent to 10 mcg of estradiol. Vagifem was the first of this class of product to be shown effective for relief of vaginal symptoms and came out in the United States in 2010.470 The tablets are packaged in individual vaginal applicators and used nightly for 14 nights, followed by twice weekly maintenance therapy.
While the 10 mcg tablet can be useful for patients who need to minimize systemic absorption of estrogen (e.g. women with breast cancer), and is significantly more effective than placebo therapy, it appears to be slightly less effective than the original 25 mcg dose (now unavailable) for initially inducing epithelial cell maturation, providing relief of vaginal symptoms, decreasing pH, and improving urogenital atrophy.471 The use of three times weekly dosing for a total of 30 mcg weekly is helpful in cases where twice-weekly Vagifem 10 mcg is not sufficient. Yuvafem is the name of a generic form of Vagifem and is used in the same way. It is usually less expensive. The typical serum estradiol level (pg/mL) is 5.5 (10ug insert).
Imvexxy is another vaginal estrogen treatment option approved for moderate to severe dyspareunia due to menopause.472 Imvexxy is an estradiol soft gel vaginal insert that offers an applicator-free experience and any time-of-day dosing, which may help support patient compliance.473 The vaginal inserts are applied daily for 14 days followed by twice weekly administration. Imvexxy has the only ultra-low dose vaginal estradiol as it is available in both 4 mcg and 10 mcg doses.474 475 476 Typical serum estradiol level (pg/mL) is 3.6 (4 ug insert) and 4.6 (10 ug insert).
Vaginal ring
A silastic ring impregnated with estradiol (Estring, Phadia) delivers 7.5 mcg of estradiol locally to the vagina daily for a period of three months.477 At that time, the woman or clinican replaces it with a new ring. The estradiol-releasing vaginal ring with its 3 months of action does improve adherence.
The product has proven efficacy in multiple placebo-controlled RCTs,478 479 480 providing relief of genitourinary symptoms, including dyspareunia, dysuria, and urge incontinence. Serum estradiol levels with use of the low-dose vaginal ring remained in the postmenopausal range (from 5 pg/mL to 10 pg/mL). 481 The risk of VTE was not increased in a 2020 systematic review of RCTs and three large observational studies.482
Patients should be encouraged to remove and replace their own rings if technically possible. The ring can remain in the vagina during sexual activity but may change position, occasionally tightly encircling the cervix, or dislodge with bowel movements, Valsalva maneuvers, douching, or vaginal sexual penetration. In some women, especially those with vaginal prolapse, retention of the ring may be difficult.
Another vaginal ring, (Femring), releases systemic levels of estradiol acetate (50 to 100 mcgs per day) and is not recommended for treatment of vulvovaginal atrophy (GSM) alone.
Vaginal estrogen absorption and need for progestogen
The question of possible need to use progestogen in conjunction with local vaginal estrogen therapy remains an area of controversy. Opinions range from a belief that progestogen is needed with any kind of vaginal estrogen to the belief that progestogen offers little, if any benefit and adds significant risk. Progestogen therapy can expose the patient to side effects including drowsiness, swelling, and breast pain (more common with DMPA/DepoProvera), acne and hirsutism (more common with norethindrone), dizziness or fatigue, or adverse mood effects.
The controversy revolves around the theoretical risk of systemic estradiol absorption. Commonly used vaginal estrogen regimens (7.5 mcg ring, 10 mcg tablets, and 0.3 mg CCE cream) were previously thought to yield minimal increases in serum estradiol concentrations (within the normal postmenopausal range).483 484 The estradiol assays used for these studies, however, were not sufficiently sensitive for accurate assessment of systemic absorption.485 Newer, highly sensitive assays have detected increased estradiol levels, but not to premenopausal levels.486
A recent Medline and Pubmed search showed that low dose vaginal estrogen, arbitrarily defined as the 7.5 mcg vaginal ring and the 10 mcg vaginal tablet, increases plasma estradiol levels during chronic administration, but not above the normal postmenopausal range of<20 pg/ml. Intermediate doses of 0.3 mg conjugated equine estrogen cream result in plasma estradiol levels approaching or exceeding 20 pg/ml. 487
The North American Menopause Society, in its 2020 position statement on the treatment of GSM, concluded, that based on available limited safety data, use of a progestogen488 489 and routine endometrial surveillance490 491 492 are not recommended in low-risk women using low-dose vaginal ET. Women at increased risk of endometrial cancer because of obesity or diabetes may warrant endometrial surveillance. Any patient on vaginal estrogen therapy who experiences spotting or bleeding requires a thorough evaluation that may include transvaginal ultrasound (TVU) and/or endometrial biopsy.493
Other experts define low dose topical estrogen as ≤0.5 g conjugated estrogen or ≤50 mcg estradiol, and state that a progestogen is probably not necessary to protect against endometrial hyperplasia in women receiving daily, local, low dose estrogen (0.3 mg conjugated estrogen) for vaginal atrophy. A progestogen should be taken when estrogen doses above these levels are used.494 No cases of atypical endometrial hyperplasia or endometrial carcinoma causally associated with the use of any local estrogen have been reported in the literature.495
Another systematic review of 20 RTCs on the use of isolated vaginal estrogen for 12-52 weeks in postmenopausal women, showed the rate of endometrial cancer and hyperplasia to be 0.03% and 0.4% respectively. Limited available studies indicate no clear endometrial proliferation although systematic endometrial biopsies were not performed.496
Still other experts suggest individualization of the decision to use progestogen during low-dose vaginal estrogen therapy.497 While measurement of endometrial thickness is widely used for monitoring during use of local estrogen, reliability and accuracy are not yet documented. Studies of the endometrial effects in women who have been treated with low dose vaginal estrogen therapy for longer than one year have not been done, but should be undertaken.
ACOG and NAMS advise that low-dose vaginal estrogen can be used indefinitely and do not advise discontinuation at a specifc age.498 499
Estrogen replacement therapy and Candida
Estrogen provides a high glycogen content in the vaginal environment, yielding an excellent carbon source for Candida growth and germination. Estrogen also enhances the ability of the vaginal epithelial cell to adhere to Candida, leading to invasion of the cell and infection. A receptor or binding system for female reproductive hormones has been documented in Candida cells.500
It is uncommon for postmenopausal women not on hormone therapy to experience yeast infection. In contrast, it is possible for a woman to be yeast-free until the commencement of hormone replacement therapy at menopause. In the postmenopausal woman, it is essential to document Candida by microscopy and culture since the differential diagnosis of itching, burning, and erythema includes atrophy, dermatosis, and vulvodynia. Recurrent yeast (more than three documented positive cultures in a year) may require adjustment of estrogen use to a lower level, discontinuation of estrogen therapy, or suppression with fluconazole 150 mg weekly.
Table P-24: Local treatment of vaginal atrophy with estrogen, lowest to highest doses501
| Imvexxy inserts, 4 mcg or 10 mcg intravaginally for 14 days, then twice weekly. (Total 8 mcg to 20 mcg/week) |
| Vagifem tablets, 10 mcg intravaginally nightly for 14 days, then twice weekly (total 20 mcg/week) |
| Estring, vaginal ring, 7.5 mcg/24 hr inserted vaginally once every 90 days (total 52.5 mcg/week) |
| Estrace cream, (100 mcg estradiol/1 gm cream): 0.5 gm intravaginally, nightly for seven days, then twice weekly (total 100 mcg/wk) |
| Premarin cream, (0.625 mg or 625 mcg conjugated estrogens/1 gm cream): 0.5 gm intravaginally, nightly for seven days, then twice weekly (total 600 mcg/wk) |
Selective estrogen receptor modulators
Selective estrogen receptor modifiers (SERMS) use their varied effects on alpha and beta estrogen receptors to produce unique therapeutic effects. Ospemifene, a SERM, is an oral agent appproved for the treatment of women with moderate to severe dyspareunia associated with VVA.502 Daily use of the 60 mg pill improves the vaginal maturation index, vaginal pH, and symptoms of GSM.503 The most common side effects of ospemifene are hot flashes and urinary tract infections.504 Ospemifene treatment can be associated with significantly greater endometrial thickness after one year of use.505
Ospemifene labeling states that the drug should not be used in women with known or suspected breast cancer because it has not been adequately studied in this group. However, its use has been associated with a decreased risk for breast cancer and breast cancer recurrence in preliminary studies.506 Clinical studies indicate that ospemifene improves bone markers significantly more than placebo and comparable to oral estrogens and other SERMs approved for osteoporosis treatment and prevention. In women with vaginal dryness and low bone density, ospemifene may offer an advantage over locally applied estrogens.507 Ospemifene may also be a superior choice in patients who cannot tolerate local medications (such as patients with Bechet’s). Patient preference is also a consideration.
Not all SERMs have the same clinical effects on GSM. Tamoxifen and raloxifene do not improve vaginal symptoms. Duavee (bazedoxifene in combination with oral conjugated equine estrogens) does improve symptoms of vaginal atrophy but the improvement in symptoms may be related to the equine estrogens alone.
Vaginal androgen therapy
Androgens contribute to the maintenance of genitourinary tissue structure and function. The effects of androgens can be distinct from those of estrogens or can be complementary. Recent research has shown that androgen-mediated processes may be involved in the full or partial resolution of GSM in women.508
Vaginal prasterone
A daily intravaginal insert of DHEA/prasterone was approved in 2016 for the treatment of dyspareunia secondary to moderate to severe vulvovaginal atrophy. DHEA is an endogenous steroid, converted by aromatase activity at the intracellular level into androgens and estrogens. The suppository is designed to be placed in the vagina nightly.
Compared to placebo, vaginal prasterone for 12 weeks was associated with improvement in dyspareunia, pH, and vaginal maturation and there were no endometrial changes.509 Circulating steroid levels remained within the normal range for postmenopausal women.510511
A retrospective cohort analysis showed women treated with prasterone had a significantly lower incidence of UTI compared to those with untreated GSM.512 Recent expert opinion suggested that intravaginal DHEA may be effective in the treatment of other types of sexual dysfunctions, such as decreased lubrication, decreased sexual desire, and decreased sexual satisfaction. Further studies, which consider alternative dosing and indications, are pending.513
Breast cancer risk has not been fully evaluated for vaginal DHEA, although the FDA-approved labeling for this intervention lists breast cancer as a warning, without a contraindication.
Vaginal testosterone
Intravaginal testosterone has been studied in short-term interventions (4-12 weeks). Fernandes et al randomized women aged 40-70 to receive one of these vaginal treatments: conjugated estrogens, testosterone proprionate, or placebo (glycerin lubricant) applied three times a week for 12 weeks. Both testosterone and estrogen treatments reduced the pH to <5 and increased the vaginal cell score and the number of lactobacilli. Analyses of serum hormone levels at 6 and 12 weeks showed no significant differences between treatment and placebo groups with no changes in endometrial thickness observed in any of the groups.514 A meta-analysis concluded that the effect of vaginal testosterone on sexual function and sexuality scores were similar to that of estrogen therapy.
In breast cancer survivors taking aromatase inhibitors, sexual interest, sexual dysfunction, and satisfaction had more improvement in the group receiving testosterone compared to the group receiving estrogens.515 However, dyspareunia or vaginal dyness were not assessed. Longer and larger studies are needed to assess the safety and efficacy of this therapy.516
Lidocaine
Another nonhormonal option for women with severe penetrative dyspareunia is the use of topical lidocaine. Over the counter, as well as prescription preparations, are available at varying strengths. In one study, 4% aqueous lidocaine applied to the vulvar vestibule before vaginal penetration resulted in significant improvements in dyspareunia and sexual function.517
Patients should be instructed to fully saturate a cotton ball (or more than one depending on size) or a cotton swab with 4% or 5% aqueous lidocaine and then hold the cotton ball or swab against the vulvar vestibule for three minutes before vaginal penetration takes place.518
Direct applicaton of lidocaine may result in discomfort (burning), so lower strengths and dilution in a water-based lubricant may be recommended initially. Lidocaine may affect the partner’s sensation. Allergic-like reactions have been reported but are rare.
Pelvic floor rehabilitation and vaginal dilators
Pelvic floor physical therapy is well established in the treatment of urinary incontinence and may be used as an adjuvant treatment for urinary symptoms of menopause. More recently, pelvic floor muscle training has been shown in studies to significantly reduce symptoms of vulvovaginal atrophy in postmenopausal women, as well. Mercier, et al demonstrated that a 12 week program by physical therapists increases vaginal wall lubrication, thickens the vaginal epithelial surface, and improves vaginal color. The decrease in VVA signs and urinary symptoms is therorized to be related to improved blood flow in the arteries supplying vulvovaginal tissues, improved pelvic floor muscle relaxation capacity, and increased vulvovaginal tissue elasticity.519 Starting pharmacologic tratment ot restore tissue integrity before initiating pelvic floor physical therapy may facilitate progress.
Vaginal dilators of graduated sizes can play an important role in restoring and maintaining vaginal function for penetration. Vaginal dilators primarily provide the fearful woman, traumatized by atrophy-related dyspareunia, with a way to trial treatment effects by, in the privacy of her home, inserting graduated dilators to test for pain and gain in confidence that treatment is working. Dilators may restore vaginal capacity by expanding the vagina in width and depth allowing for comfortable sexual activity. They are also useful for vaginal stenosis, vaginismus (pelvic floor myalgia), and after pelvic radiation therapy. See Annotation L for more on the use of dilators.Annotation L: click on Vaginal Dilators
This article from Sloan Kettering provides helpful instructions for patients in how to use dilators:
https://www.mskcc.org/cancer-care/patient-education/how-use-vaginal-dilator
Diltors may be washed with warm water and mild soap. Silicone dilators may be sterilized by boiling for 5 minutes or by autoclave. GRS (gender reassignment surgery) and Rigid Plastic dilators can NEVER be boiled or autoclaved. Lubricant use with dilators may have some restrictions (e.g., silicone lubricants should not be used with silicone dilators). There are many types of dilators available. The authors use Soul Source dilators manufactured in the United States or those available at Vaginismus.com https://www.vaginismus.com/ where they are called “inserters.”
Energy-based therapies
The US Food and Drug Administration (FDA) has licensed the CO2 laser systems for “incision, excision, ablation, vaporization, and coagulation of soft tissues in the body.” These devices have been used in specialties such as dermatology, ENT, urology, neurosurgery, and gynecology since 2010. FDA authorized indications for energy-based devices for gynecology include the destruction of abnormal cervical or vaginal tissue, condyloma, and precancerous lesions. Since then, many health professionals, physicians, and nonphysicians have adopted using such energy-based devices without a precise indication, including the promise of genital “rejuvenation.”
Vulvovaginal energy-based devices, including lasers (fractional CO2, Erbium: YAG and radio-frequency devices) are under investigation as treatments for GSM, but none have FDA approval for this indication. In a 2018 Safety Communication, the FDA issued a public warning about the use of these devices for vaginal cosmetic purposes, stating that the effectiveness and safety of the devices have not yet been established.520
The main benefits of energy-based device use in the vagina are purported to be as follows: a) thickening of the non-keratinized epithelium, b) increase in local angiogenesis, c) improvement in lubrication, d) normalization of the genital microbiome, e) decline in vaginal pH, f) development of new collagen fibers and restoration of vaginal architecture, improvement in elasticity and decrease in the frequency of microtraumas, and g) improvement in genitourinary symptoms.521
Adverse events associated with energy-based therapies include discomfort during treatments, vaginal scarring, vaginal lacerations on resumption of intercourse, and persistent and/or worsening dyspareunia.522 The recommended course of therapy includes three treatments which are costly and generally not covered by insurers. The effect of the treatment seems to be transitory for most women and repeat courses of treatment are often necessary.
Consensus statements regarding the use of energy-based therapies for GSM treatment have been published by several professional societies summarizing the small but growing body of evidence, and expressing concerns about safety.523 524 525 Currently, the International Society for the Study of Vulvovaginal Disease (ISSVD) does not endorse the use of these technologies out of the setting of clinical trials.526 The American College of Obstetricians and Gynecologists’ (ACOG) 2021 Clinical Consensus No.2 on the treatment of urogenital symptoms in women with estrogen-dependent breast cancer states: “Laser therapy has shown promise in a research setting, with small trials.”527
Data from a recent meta-analysis suggests that CO2 laser is a safe energy-based therapeutic option for the management of VVA and/or GSM symptoms in postmenopausal women; however, the quality of the body of evidence found was “very low” or “low.”528 Until well-designed RCTs versus placebo or other interventions are available, these procedures should be cautiously used for the treatment of GSM.
In the absence of adequate clinical trials, patients who desire these treatments should be referred to a practitioner with extensive experience performing the procedure. If energy-based technology is offered to the patient as a treatment option, the indication for the procedure should be conveyed in a clear and transparent way. An explanation on the limitations and risks of the method should also be proviced.
Treatment of women with breast cancer
Many patients and clinicans remain hesitant to use any estrogen-related compounds in this patient population. In addition, adjuvant treatments (aromatase inhibitors (AIs) or tamoxifen) work by either lowering estrogen concentrations or antagonizing estrogen effects. For pre-and post-meopausal patients receiving these treatments, the vulvovaginal symptoms that result from the effect of estrogen loss may be negatively life-altering. Fortunately, most new data do not show an increased risk of cancer recurrence with vaginal estrogen use among women currently undergoing treatment for breast cancer or those with a personal history of breast cancer. However, in a study of postmenopausal patients with a history of early estrogen-positive breast cancer treated with aromatase inhibitors (AI), those who used vaginal estrogen after the breast cancer diagnosis had a higher risk of breast cancer recurrence compared with nonusers (adjusted hazard ratio 1.39, 95% CI 1.04-1.85), an effect not seen in patients treated with tamoxifen. The authors of the current UpToDate article on Treatment of VVA, therefore, only prescribe vaginal estrogen for GSM to their patients being treated with tamoxifen, not AI. 529
Breast cancer patients with GSM may benefit from nonhormonal therapies including lubricants, moisturizers, and pelvic floor physical therapy. If symptoms persist, however, other therapies may offer relief and should be offered, including topical lidocaine, low-dose vaginal ET, vaginal DHEA, ospemifene, and possibly vaginal energy-based therapies.530 531 532 533
According to FDA labeling, even low-dose ET is contraindicated in women with breast cancer. Off-label use of several products can be employed because of their very low systemic absorption.534 Low dose vaginal ET formulations, including the estradiol tablet, insert, and ring, result in serum estradiol within the postmenopausal range and similar to placebo blood levels.535 536 Several organizations, including the American College of Obstetricians and Gynecologists and the North American Menopause Society, have endorsed the use of low-dose vaginal estrogens in women with breast cancer, including ER-positive disease. Based on a recent meta-analysis,537 the International Society for the Study of Vulvovaginal Disease (ISSVD) concluded that vaginal estrogen use was not associated with significant absorption, which provides indirect evidence of safety.538
Use of vaginal DHEA for GSM in women with breast cancer is not contraindicated according to the FDA, but US labeling advises caution because estrogen is a metabolite of DHEA.539 Although vaginal DHEA has not been studied in women with a history of breast cancer, levels of estradiol and testosterone remain within the postmenopausal range in non-breast cancer afflicted women.540
Ospemifene is not recommended for treatment of GSM in women with known or suspected breast cancer because the drug has not been adeuately studied in this group.
Clinical trials of energy-based therapy (laser) for GSM in survivors of breast cancer provide limited evidence for safety and efficacy in this patient population.
In all women with a history of or high risk for breast cancer, but especially those on AI, estrogen preparations with the lowest absorption such as the 4 mcg capsule or the 10 mcg tablet or capsule are a reasonable choice. The fact remains that a safe level of serum estrogen in relation to breast cancer risk is not known for women on or off adjuvant hormonal treatment, while quality of life concerns are important to these women. Shared decision making and involvement of the patient’s oncologist are important.
In breast cancer survivors with dyspareunia and not using vaginal estrogen, exquisite pain can sometimes be elicited with simple touch of the vulvar vestibule. This pain may be attenuated with pre-coital application of aqueous lidocaine 4%. Vaginal tenderness was rare despite severe atrophy.541
The American College of Obstetricians and Gynecologists (ACOG) has made the following recommendations:542
- Nonhormonal approaches are the first-line choices for managing urogenital symptoms or atrophy-related urinary symptoms experienced by women during or after treatment for breast cancer.
- Among women with a history of estrogen-dependent breast cancer who are experiencing urogenital symptoms, vaginal estrogen should be reserved for those patients who are unresponsive to nonhormonal remedies.
- The decision to use vaginal estrogen may be made in coordination with a woman’s oncologist. Additionally, it should produced by an informed decision-making and consent process in which the woman has the information and resources to consider the benefits and potential risks of low dose esterogen.
- Data do not show an increased risk of cancer recurrence among women currently undergoing treatment for breast cancer or those with a personal history of breast cancer who use vaginal estrogen to relieve urogenital symptoms.
Estradiol can be compounded in any desired strength.
Natural and bio-identical hormones
Women assume that “natural” hormones–any products whose principal ingredient has an animal, plant, or mineral source–would be better or safer. The term “natural” is confusing, sometimes referring to source of preparation from a plant or animal, sometimes referring to chemical structure identical to human.(Premarin is natural estrogen, but it comes from horse urine and is horse estrogen). 543 The only true natural estrogens are those manufactured in the human female body: estrone, estriol, and estradiol. These are not available commercially. In addition, “natural” does not necessarily guarantee safety since many natural products are unregulated.
“Bioidentical” is a term referring to products that contain hormones chemically identical to those found in humans. The interest in a more natural approach to hormone therapy has focused attention on bioidentical hormones. These are not found in nature but are made, or synthesized, from a plant chemical extracted from yams and soy.544 A hormone is bioidentical when it has the exact structure of estrone, estriol, or estradiol, whether it comes from the ovary or is extracted from plants. Multiple specially compounded formulae have no advantage over commercially manufactured, FDA approved estradiol except if special dosage is required or when a special vehicle is needed in the case of particularly sensitive women.
Desquamative inflammatory vaginitis
Introduction
Desquamative inflammatory vaginitis (DIV) is a newly recognized clinical syndrome characterized by persistent purulent vaginal secretions and vaginal erythema; submucosal cervicovaginal petechiae are frequently noted, accompanied in some patients, by irritative vaginal symptoms, and/or dyspareunia. 545 The cardinal feature of DIV is inflammation called aerobic vaginitis by Donders after the term aerobic vaginitis was introduced in 2002 in reference to the abnormal vaginal microbiome defined as CST IV. 546 The term desquamative inflammatory vaginitis is attributed to Gray and Barnes from 1965. 547
Pathophysiology
Inflammation causes disruption of vaginal cell wall maturation process, which, in turn, causes epithelial cell shedding and the typical increased parabasal cells seen on microscopy. Loss of normal lactobacilli leads to colonization with other opportunistic bacteria, but no infectious cause for DIV has been found. 548 549
Etiology
The etiology remains unknown. It appears to be a dysbiosis of the normal vaginal microbiome associated with inflammation. In desquamative inflammatory vaginitis, the vagina is colonized with facultative bacteria, not the obligate anaerobic bacteria that colonize the vagina in bacterial vaginosis. The microflora in desquamative inflammatory vaginitis typically consist of Escherichia coli, Staphylococcus aureus, group B streptococcus, or Enterococcus faecalis. 550
The microbiome associated with desquamative inflammatory vaginitis is less well understood than the bacterial vaginosis microbiome. Desquamative inflammatory vaginitis may also represent a systemic inflammatory syndrome that produces vaginal inflammation, resulting in abnormal vaginal flora. As with bacterial vaginosis, understanding the mechanism underlying the loss of vaginal lactobacilli should shed light on the pathogenesis of desquamative inflammatory vaginitis. 551
While it is well documented that clinical signs and symptoms of DIV can be seen in women with lichen planus, experts around the world now agree that DIV also exists as a distinct clinical entity in the absence of lichen planus.552 A number of clinicians who treat women with DIV have reported an association with other autoimmune disease 553 including thyroid disease, 554 and inflammatory bowel disease, including Crohn’s.555, 556 Vitamin D deficiency has also recently been proposed as a possible etiology of DIV.557 The dysbiosis associated with DIV presents an unclear relationship with the DIV condition itself.. The essential question is whether inflammation, tissue erosion, and subsequent exposure of deep layers of epithelial cells are triggered by specific bacteria or, whether the inflammatory environment is unfavorable to lactobacilli- as a result, other bacteria gain terrain. 558
DIV can occur at any age, before or after menopause. Most women with DIV are Caucasian.559 The average age of women with DIV in several studies was about the mid-40s.560 561 Among women presenting to a specialty clinic with complaints of chronic vaginitis, the incidence of DIV was 8%, ranging from 5% in women <50 years old to 15% in women over 50. 562,563
Clinical manifestations
Classic symptoms of DIV include excessive pus-like yellow-green discharge that may have been present for months or years.564 The discharge may be blood stained and may be associated with vulvar burning, irritation and/or itching. Intercourse is often uncomfortable or painful. Few women complain of odor. The Pap smear may be abnormal (ASCUS) because of inflammation of the vagina and cervix. Women often report having been treated repeatedly with antibiotics, anti-fungals, and estrogen without improvement.
Diagnosis
Diagnosis is made by the typical findings of yellow-green purulent vaginal discharge, an elevated vaginal pH (>5.0), the presence of parabasal cells with elevated numbers of leukocytes and loss of lactobacilli. A generalized or patchy vaginal inflammation is present in most patients high in the vaginal vault, with reversible ecchymotic spots also seen in some patients at the introitus. Erythematous spots (“strawberry patches”) can also be seen on the cervix of women with DIV, in absence of trichomoniasis. Inflammation and tenderness in the vestibule often accompany DIV. 565 When taking a sample for pH, do not forget that the pH of cervical secretions is normally elevated, in contrast with secretions taken from the lower part of the vagina.
Desquamative inflammatory vaginitis in its early development may present with the complaint of increased, irritating discharge that has not yet developed to the purulent yellow-green stage. Vaginal tenderness and pain with thrusting may be present. There may be an occasional parabasal cell on wet mount with a diminished, not absent, number of lactobacilli. Women may complain of increased vaginal discharge for years with mild dyspareunia before clear findings by wet mount are present. We have also had the experience of seeing normal wet mounts alternating with mild abnormalities in early disease.
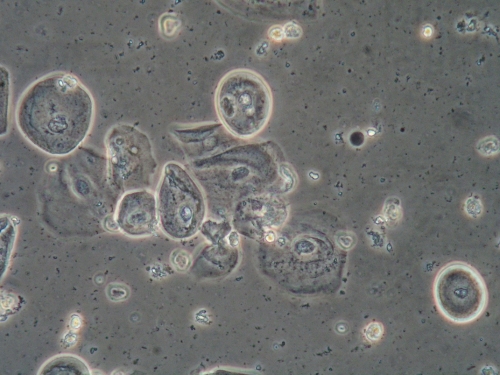
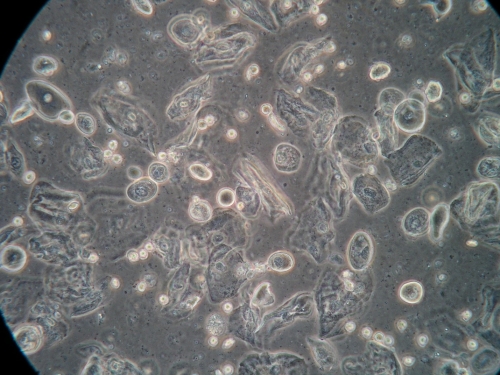
Differential diagnosis
DIV is often misdiagnosed as bacterial vaginosis because of the presence of discharge and odor, elevated pH and the interpretation of central bacterial stippling of epithelial cells as clue cells. Another inflammatory vaginitis called Aerobic Vaginitis (AV) also disrupts the lactobacillus leading to increased pH, and in extreme cases appears the same as DIV,566 and is treated with local antibiotics. DIV is also misdiagnosed as trichomoniasis because of the discharge and inflammation, erythematous cervical patches, elevated pH and presence of multiple white blood cells on wet mount. Testing for gonorrhea and Chlamydia needs to be done, and cultures for trichomoniasis and Group A streptococcus should be sent. Changes in the wet prep that can mimic “early DIV,” can be caused by Candida, so yeast culture needs to be done even if it is not seen on wet prep. The syndrome of lichen planus needs to be ruled out by observing over time for scarring with synechial formation or telescoping in the vagina. In peri- or postmenopausal women, vaginal estrogen should be tried first, to rule out atrophy that may cause similar symptoms and wet mount; in the case of DIV, the use of local estrogen alone will not resolve the symptoms.
Treatment
Treatment for DIV is with either intravaginal steroids (intravaginal hydrocortisone 100mg vaginally, made up by a compounding pharmacy as a suppository or cream and inserted nightly for 14 days, then every other day for 14 days, and then as needed for suppression of symptoms) or intravaginal clindamycin (vaginal clindamycin 2% gel is inserted nightly for 14 days). 567The clindamycin is used for its significant anti-inflammatory effect, not because DIV is infectious. Many clinicians use one modality first, then the other. Some experts use the combination intravaginal hydrocortisone from 100-400 mg/gm with clindamycin 2%, and estradiol 0.01%. In any case, at least two weeks of nightly treatment- and sometimes more- are needed to control the initial inflammation and improve the patient’s symptoms. Then a maintenance program is devised. In a series of 130 women diagnosed with DIV in Michigan, median treatment length was 8 weeks; 25% women were cured at one year with 55 women reporting that their symptoms were well controlled with maintenance therapy and 15 women reporting only partial control.568 Similar findings from a series of women treated for DIV in Pennsylvania and Israel suggested a 41% cure rate after clindamycin therapy for two weeks, with 32% of patients having recurrences that finally cleared with re-treatment and 27% of patients requiring maintenance therapy.569 In our own experience with patients in Massachusetts, DIV often requires lengthy treatment of 4-12 weeks and then maintenance therapy (with hydrocortisone or clindamycin once or twice a week) to prevent recurrence. It is difficult for women to understand the chronicity of a type of condition that is usually cured with a tube of cream; many discontinue treatment several times before being comfortable with the concept of management without cure.
It has also been our experience that vaginal clindamycin causes significant burning for some women. This may result from the vaginal inflammation, or may represent a contact irritant reaction; in these cases, use of hydrocortisone first may be necessary. Hydrocortisone 100 mg suppositories may also cause burning initially; in these cases, we ask women to cut the suppository in half and initiate treatment with 50 mg every other day, building up gradually to 100 mg. Protecting the epithelium of the vestibule with a layer of vaseline may help with the burning. In severe cases, a woman may not tolerate topical treatment and may require a depot injection of triamcinolone 60 mg IM. (LINK, Annotation ?). The injection may be repeated in one month, or if the wet mount shows a significant decrease in neutrophils and lymphocyctes and parabasal cells, the patient may resume topical hydrocortisone therapy.
Management should include periodic re-evaluation, monthly at first, then less frequently. By history, the inflammation and soreness lessens, the discharge dissipates, (but there is always residue from suppositories). Dyspareunia is the last symptom to resolve, taking weeks to months. In severe cases, a woman may need a centrally acting agent such as a tri-cyclic antidepressant or gabapentin, as well as physical therapy to the pelvic floor to combat the learned response to pain. (LINK, Annotation K: Vulvar pain and provoked or unprovoked vulvodynia). The wet mount shows gradually decreasing numbers of white blood cells and parabasal cells. The lactobacillus gradually returns, correcting the pH.
Lichen planus
Lichen planus can affect the vagina, leading to abnormal vaginal discharge discussed under abnormalities of the epithelium in Annotation O (LINK). Vulvar lichen planus is discussed in the Atlas of Vulvar Disorders.
Vaginal cancer
Vaginal cancer may present with a blood-tinged discharge. (Annotation O: The vaginal epithelium).
Sexually transmitted infections: For the most up to date information on STIs, see the CDC website. (LINK, www.cdc.gov//std/treatment/2010/vaginal-discharge.htm
Cervicitis, Cervical Polyps, Cervical Ectropion: See Annotation M: Speculum exam and examination of the cervix
Vaginal lesions, Vaginal Polyps, Granulation Tissue: See Annotation N: The vaginal architecture
Fistula: See Annotation N: The vaginal architecture
Graft versus host disease: See Annotation O: The vaginal epithelium
Sjögren’s syndrome: See Annotation O: The vaginal epithelium
Crohn Disease: See Annotation O: The vaginal epithelium
Introduction
With a complaint of vaginal discharge, upper tract disease is a consideration, although not one’s first thought unless fever and pelvic pain are present. If an initial diagnosis is not responding to treatment or if a case is considered recalcitrant, upper tract disease must be ruled out. Upper genital tract disease is difficult to diagnose because presentation, symptoms, and signs vary widely and because many women have subtle or mild symptoms.570
Acute endometritis and pelvic inflammatory disease
Pelvic inflammatory disease (PID) is a primary concern. This involves infection of the upper female genital tract by upward spread of infectious microorganisms through the cervix to the uterus, fallopian tubes, ovaries, and peritoneal cavity, leading to an array of inflammatory disorders, including endometritis, parametritis, salpingitis, oophoritis, peritonitis, perihepatitis, and tubo-ovarian abscess (TOA).571 Although multiple organisms can be responsible – Chlamydia and gonorrhea are common causes – the specific agent is often not identified.572
Epidemiology and risks
Difficulties with PID surveillance arise because a cheap, simple, and accurate diagnostic test does not exist. Problems with case definition and diagnostic accuracy lead to challenges with data, and inability to assess trends in PID prevalence with certainty.573 Currently, hospitalizations for acute PID have dropped from >70,000 women aged 15-44 in 1998 to 45,000 in 2007.574 Initial visits for PID to physicians’ offices by women aged 15-44 have dropped from >240,000 in 2000 to 120,000 in 2009.575
Behaviors such as early sexual debut, multiple sexual partners, unprotected sexual intercourse, and drug use affect the sexually transmitted disease (STD) and PID rates. Exposure to sexually transmitted infection (STI) by Chlamydia trachomatis and Neisseria gonorrhoea is a major risk factor for the acquisition of PID, but trends in PID cannot be inferred from surveillance data of either infection.576 Vaginal douching, a common behavior, particularly among African-American women, may facilitate the ascension of organisms to the upper genital tract. Studies have noted that vaginal douching within 6 days of being diagnosed with PID is associated with endometritis and upper genital tract infections.577
Pathogenesis
PID is a mixed, polymicrobial infection in the upper genital tract. It begins as an infection of the cervix with ascension of microorganisms to the upper genital tract. It is theorized that offending organisms gain access to the endometrium by attaching to the columnar epithelium of cervical ectropion during the menses or via movement of spermatozoa. Viruses such as HIV or herpes simplex also can facilitate this process by disrupting immunologic barriers to infection. The inflammatory response to offending organisms disrupts the normal host defense mechanisms by altering the vaginal pH, normal vaginal flora, and the cervical mucous barrier.578
Clinical manifestations
The clinical presentation of PID varies from asymptomatic to severe disease. Acute midline or bilateral lower abdominal pain is the most common presenting symptom, and one not usually seen with other vulvovaginal conditions. Such pain in the vulvovaginal patient always prompts investigation. Associated symptoms include yellow or malodorous vaginal discharge, dyspareunia, irregular vaginal bleeding, dysuria, nausea, vomiting, and fever. The severity of PID depends on the duration of symptoms and the etiologic agent; PID associated with C. trachomatis tends to cause less acute symptoms than does PID associated with N gonorrhoeae.579
Diagnosis
No diagnostic indicators have been found to predict pelvic inflammatory disease reliably.580
The findings upon physical examination include moderate-to-severe lower abdominal tenderness with or without rebound tenderness. The pelvic examination findings vary, but the majority of women with PID have either mucopurulent cervical discharge or evidence of WBC on a microscopic evaluation of a saline preparation of vaginal fluid. If the cervical discharge appears normal and no WBCs are observed on the wet prep of vaginal fluid, the diagnosis of PID is unlikely, and alternative causes of pain should be investigated.581 A wet prep of vaginal fluid offers the ability to detect the presence of concomitant infections (e.g., bacterial vaginosis and trichomoniasis).
Laparoscopy can be used to obtain a more accurate diagnosis of salpingitis and a more complete bacteriologic diagnosis. However, this diagnostic tool frequently is not readily available, and its use is not easy to justify when symptoms are mild or vague. Moreover, laparoscopy will not detect endometritis and might not detect subtle inflammation of the fallopian tubes. Consequently, a diagnosis of PID usually is based on clinical findings.582
Empiric treatment of PID should be initiated in sexually active young women and other women at risk for STIs if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than PID can be identified, and if one or more of the following minimum criteria are present on pelvic examination:
- cervical motion tenderness
- uterine tenderness
- adnexal tenderness583
Differential diagnoses
Appendicitis, cholecystitis, constipation, gastroenteritis, hernia, inflammatory bowel disease, cystitis, pyelonephritis, nephrolithiasis, urethritis, corpus luteum cyst, dysmenorrhea, ectopic pregnancy, endometriosis, ovarian cyst, ovarian torsion, ovarian tumor
Treatment
Clinicians are encouraged to follow the current CDC treatment guidelines: (Link CDC)
The following criteria for hospitalization are suggested:584
- surgical emergencies (e.g., appendicitis) cannot be excluded;
- the patient is pregnant;
- the patient does not respond clinically to oral antimicrobial therapy;
- the patient is unable to follow or tolerate an outpatient oral regimen;
- the patient has severe illness, nausea and vomiting, or high fever; or
- the patient has a tubo-ovarian abscess.
Endometrial polyps
Introduction
Uterine polyps are growths attached to the inner wall of the uterus; these can protrude into the uterine cavity or through the cervix into the vagina.
Pathogenesis
Overgrowth of cells in the lining of the uterus (endometrium) leads to the formation of uterine polyps. The cause of the overgrowth is not known. The size of uterine polyps ranges from a few millimeters — no larger than a sesame seed — to several centimeters — golf ball sized or larger. They are attached to the uterine wall by a large base or a thin stalk.
Epidemiology
Endometrial polyps are diagnosed in 10-40% of women with abnormal uterine bleeding. 1-12% of asymptomatic women are found to have endometrial polyps on routine gynecologic examinations. 585
Clinical manifestations
Since most polyps are small, they probably do not often cause symptoms, and they are not a common cause of vulvovaginal complaints. Smaller polyps appear to regress spontaneously in some cases.586 However, when symptoms do occur, they usually include excessive bleeding during a menstrual period, or bleeding in between periods, or even spotting after intercourse. Some women report a few days of brown blood after a normal menstrual period. Polyps cause these symptoms because they dangle from their stalks and irritate the surrounding tissue, which causes the tissue to rub off, exposing tiny blood vessels. These blood vessels bleed, leading to spotting or vaginal bleeding. In our experience, some women with endometrial polyps complain of abnormal vaginal discharge as well as periodic spotting and/or bleeding.
Pathophysiology
Most studies show that the prevalence of malignancy in endometrial polyps varies by age and menopausal status, and that postmenopausal women with abnormal uterine bleeding carry the highest risk of premalignant and/or malignant tissue change. The risk of malignancy in premenopausal women appears to be low.587
Treatment
Endometrial polyp resection has a high success rate in terms of symptom relief (75-100%),588 but recurrence rate is not well studied.
Tubo-ovarian carcinoma
Introduction
While carcinoma of the fallopian tube has been thought to be a rare malignancy, recent evidence suggests that tubal malignancy may occur more frequently than reported because papillary serous cancer of the ovary may originate in the fallopian tubes.589
Incidence and risks
In the past, the fallopian tube has been reported to be the least common site of origin for a malignant neoplasm of the female genital tract, accounting for 0.2 to 0.5 percent of primary female genital malignancies. This substantial underestimation of tubal malignancy is connected to the most common subtype of epithelial ovarian cancer, papillary serous carcinoma, arising from the epithelial lining of the fallopian tube fimbria. Given the existence of widespread disseminated peritoneal tumor in patients diagnosed with papillary serous ovarian carcinoma, an origin in the tube as the primary site of tumor initiation is plausible.590 An inherited mutation in BRCA1 or 2 is the only identified risk.591
Pathology
About 90 percent of fallopian tube tumors are adenocarcinomas, and one-half of these are papillary serous adenocarcinomas. Less frequent types include endometrioid, clear cell, adenosquamous, squamous cell carcinoma, sarcoma, choriocarcinoma, and malignant teratoma.592
Clinical manifestations
Vigilance and an index of suspicion are necessary for the detection of a tubo-ovarian diagnosis in a patient presenting to a vulvovaginal clinician. Vaginal discharge, intermittent, possibly profuse, clear or serosanguinous can occur. Vaginal bleeding with a negative endometrial biopsy occurs. Approximately 10 percent have abnormal cells on Papanicolaou smear but a negative work-up for endometrial or cervical cancer (e.g., negative endometrial and endocervical curettage). What is significant for these women presenting to the vulvovaginal clinician is that they may also have colicky or dull pelvic pain (secondary to tubal distention), abdominal pressure/bloating, dyspepsia, early satiety, and urinary frequency and urgency with negative urinary culture. 593
Diagnosis
Laparoscopy and tissue diagnosis are definitive. Standard testing such as CA 125 is elevated in most patients, but is not diagnostic; abdomino-pelvic computerized tomography (CT) or magnetic resonance imaging (MRI) is often not helpful. As with ovarian cancer, delay in diagnosis of fallopian tube carcinoma is common. The diagnosis is made postoperatively in the majority of cases.
Treatment
Treatment guidelines for fallopian tube carcinoma are identical to epithelial ovarian cancer. Initially surgery is performed, followed by chemotherapy.
Endometrial carcinoma
Introduction
Endometrial carcinoma is always a diagnosis to be considered when there is a question of vaginal discharge, or vaginal bleeding.
Epidemiology
Endometrial carcinoma is the most common gynecologic malignancy in the United States; approximately 42,160 cases are diagnosed annually.594
Differences in epidemiology and prognosis suggest that two forms of endometrial cancer exist: those related to and those unrelated to estrogen stimulation.595
- Type I endometrial carcinoma is estrogen-related, usually presents histologically as a low-grade endometrioid tumor, and is associated with atypical endometrial hyperplasia. These patients tend to have risk factors such as obesity, nulliparity, endogenous or exogenous estrogen excess, diabetes mellitus, and hypertension.
- Type II endometrial carcinomas appear unrelated to estrogen stimulation or endometrial hyperplasia, and tend to present with higher-grade tumors or poor prognostic cell types, such as papillary serous or clear cell tumors. These patients are often multiparous, and do not have an increased prevalence of obesity, diabetes, or hypertension. They also tend to be older than women with endometrioid tumors deaths occur.
Clinical manifestations
The cardinal symptom of endometrial carcinoma is abnormal uterine bleeding, which occurs in 90 percent of cases. 596Diagnosing abnormal bleeding is difficult, especially since there are myriad causes of vaginal bleeding and women tend to minimize the degree of bleeding or discharge that occurs. Diagnostic evaluation must be undertaken when postmenopausal women present with bright red blood, blood-tinged, pink, tan, brown, or yellow staining, or ongoing excessive vaginal discharge. The American College of Obstetricians and Gynecologists (ACOG) and the Society of Radiologists in Ultrasound (SRU) advise that either TVUS (with an endometrial thickness of ≤4 mm [ACOG] or ≤5 mm [SRU]) or endometrial sampling are effective as a first diagnostic step in women with postmenopausal bleeding.597 Despite some limitations, numerous studies have shown that the endometrium is usually adequately sampled with biopsy techniques.598 Hysteroscopy and endometrial sampling are another option. The D&C has been mostly relegated to history.
Treatment
Findings of atypical endometrial hyperplasia, carcinoma in situ or invasive endometrial carcinoma require gynecologic-oncologic consultation.
References
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004 Mar 17; 291(11):1368.
- Landers DV; Wiesenfeld HC; Heine RP; Krohn MA; Hillier SL Predictive value of the clinical diagnosis of lower genital tract infection in women. Am J Obstet Gynecol. 2004 Apr, 2004; 190:1004.
- Arbyn M, Simon M, de Sanjosé S, Clarke MA, Poljak M, Rezhake R, Berkhof J, Nyaga V, Gultekin M, Canfell K, Wentzensen N. Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: a systematic review and meta-analysis. Lancet Oncol. 2022 Jul;23(7):950-960. doi: 10.1016/S1470-2045(22)00294-7. Epub 2022 Jun 13. Erratum in: Lancet Oncol. 2022 Aug;23(8):e370. PMID: 35709810.
- Huppert JS, Hesse EA, Bernard MC, Bates JR, Gaydos CA, Kahn JA. Accuracy and trust of self-testing for bacterial vaginosis. J Adolesc Health. 2012 Oct;51(4):400-5. doi: 10.1016/j.jadohealth.2012.01.017. Epub 2012 Mar 21. PMID: 22999842; PMCID: PMC3457017.
- Morris SR, Bristow CC, Wierzbicki MR, Sarno M, Asbel L, French A, Gaydos CA, Hazan L, Mena L, Madhivanan P, Philip S, Schwartz S, Brown C, Styers D, Waymer T, Klausner JD. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2021 May;21(5):668-676. doi: 10.1016/S1473-3099(20)30734-9. Epub 2020 Nov 23. PMID: 33242473; PMCID: PMC9884536.
- Morris SR, Bristow CC, Wierzbicki MR, Sarno M, Asbel L, French A, Gaydos CA, Hazan L, Mena L, Madhivanan P, Philip S, Schwartz S, Brown C, Styers D, Waymer T, Klausner JD. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2021 May;21(5):668-676. doi: 10.1016/S1473-3099(20)30734-9. Epub 2020 Nov 23. PMID: 33242473; PMCID: PMC9884536.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004 Mar 17; 291(11):1368.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004 Mar 17; 291(11):1368.
- Tietz K, Klein S. Simulated genital tract fluids and their applicability in drug release/dissolution testing of vaginal dosage forms. Dissolution Technol. 2018:40–51; 10.14227/DT250318P40.
- Paavonen, J. Physiology and ecology of the vagina. Scand. J. Infect. Dis. 1983, 40, 31–35.
- Sobel JD. Approach to females with symptoms of vaginitis. UpToDate, 2022. www.uptodate.com
- Godley MJ. Quantitation of vaginal discharge in healthy volunteers, Br J Obstet Gynaecol. 1985; 92:73.
- Stewart EG, Spencer P. The V Book, New York, Bantam Books, 2002, 49.
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Julia MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52:S46-S52.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach, S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011 Mar 15; 108 Suppl 1: 4680-7. Doi: 10.1073/pnas.1002611107. Epub 2010 June 3.
- Verstraelen H, Vieira-Baptista P, De Seta F, Ventolini G, Lonnee-Hoffmann R, Lev-Sagie A. The Vaginal Microbiome: I. Research Development, Lexicon, Defining “Normal” and the Dynamics Throughout Women’s Lives. J Low Genit Tract Dis. 2022 Jan 1;26(1):73-78. doi: 10.1097/LGT.0000000000000643. PMID: 34928256; PMCID: PMC8719517.
- Hillier SL, Austin M, et al. Diagnosis and Treatment of Vaginal Discharge Syndromes in Community Practice Settings. Clin Infect Dis. 2021;72(9):1538.
- Nappi RE, Martini E, Cucinella L, Martella S, Tiranini L, Inzoli A, Brambilla E, Bosoni D, Cassani C, Gardella B. Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for Healthy Aging in Women. Front Endocrinol (Lausanne). 2019 Aug 21;10:561. doi: 10.3389/fendo.2019.00561. PMID: 31496993; PMCID: PMC6712495
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012 Jun 13;486(7402):207-14. doi: 10.1038/nature11234. PMID: 22699609; PMCID: PMC3564958.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach, S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011 Mar 15; 108 Suppl 1: 4680-7. Doi: 10.1073/pnas.1002611107. Epub 2010 June 3.
- Verstraelen H, Vieira-Baptista P, De Seta F, Ventolini G, Lonnee-Hoffmann R, Lev-Sagie A. The Vaginal Microbiome: I. Research Development, Lexicon, Defining “Normal” and the Dynamics Throughout Women’s Lives. J Low Genit Tract Dis. 2022 Jan 1;26(1):73-78. doi: 10.1097/LGT.0000000000000643. PMID: 34928256; PMCID: PMC8719517.
- Anahtar MN, Gootenberg DB, Mitchell CM, Kwon DS. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe. 2018 Feb 14;23(2):159-168. doi: 10.1016/j.chom.2018.01.013. PMID: 29447695.
- Ravel J, et al. Vaginal biome of reproductive age women. Proc Natl Acad Sci USA. 2011 Mar 15; 108(Suppl 1): 4680-4687.
- Edwards L, Stewart K, Venkatesan A, Correlation of the presence of lactobacilli on wet mount and the vaginal pH. Presentation at the International Society for the Study of Vulvovaginal Disease, XX World Congress, September 13-17, 2009, Edinburgh, Scotland.
- Ma, B, Forney LJ, Ravel J. The vaginal microbiome: rethinking health and diseases. Annu Rev Microbiol. 2012; 66:371-389.
- Landers DV; Wiesenfeld HC; Heine RP; Krohn MA; Hillier SL Predictive value of the clinical diagnosis of lower genital tract infection in women. Am J Obstet Gynecol. 2004 Apr, 2004; 190:1004.
- Brown H, Drexler M. Improving the Diagnosis of Vulvovaginitis: Perspectives to Align Practice, Guidelines, and Awareness. Popul Health Manag. 2020 Oct;23(S1):S3-S12. doi: 10.1089/pop.2020.0265. PMID: 32997581; PMCID: PMC7591372.
- Ledger WJ, Witkin SS. Vulvovaginal Infections. Washington, D.C., ASM Press, 2007:9.
- ACOG Practice Bulletin. May 2006;72.
- CDC STI Treatment Guidelines, 2021 for Bacterial Vaginosis; accessed March, 2024: https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Greene JF 3rd, Kuehl TJ, Allen SR. The Papanicolaou smear: inadequate screening test for bacterial vaginosis during pregnancy. Am J Obstet Gynecol. 2000;182:1048-1049.
- Center for Disease control and Prevention, STI Treatment Guidelines, 2021.https://www.cdc.gov/std/treatment-guidelines/bv.htm
- ACOG. Vaginitis in nonpregnant patients: ACOG practice bulletin number 215. Obstet Gynecol 2020;135:e1–e17
- ACOG. Vaginitis in nonpregnant patients: ACOG practice bulletin number 215. Obstet Gynecol 2020;135:e1–e17
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004 Mar 17; 291(11):1368.
- Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev 2010; 23:253–73.
- Sobel JD. Candida vulvovaginitis: Treatment. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA:UpToDate, Inc. http://www.uptodate.com. (Accessed 12/8/2022).
- Nguyen Y, et al. Management of chronic vulvovaginal candidiasis: a long term retrospective study. Australasian Journal of Dermatology 2016; doi 10.111/adj12497.
- Farr A, Effendy I, Frey Tirri B, Hof H, Mayser P, Petricevic L, Ruhnke M, Schaller M, Schaefer APA, Sustr V, Willinger B, Mendling W. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses. 2021 Jun;64(6):583-602. doi: 10.1111/myc.13248. Epub 2021 Feb 27. PMID: 33529414; PMCID: PMC8248160.
- Dignani M, Solomkin J, Anaissie E. Candida. In: Anaissie E, McGinnis M, Pfaller M, eds. Clinical mycology. USA: 2009; Elsevier, 197–231.
- Sobel J, and MItchell, C. Candida vulvovaginitis: Clinical manifestations and diagnosis. UpToDate, Inc. 2022
- Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34:7–14.
- The Medical Letter on Drugs and Therapeutics, 6 September 2021. Ibrexafungerp (Brexafemme) for Vulvovaginal Candidiasis. 63(1632):141.
- The Medical Letter on Drugs and Therapeutics, 8 August 2022. Oteseconazole (Vivjoa) for Recurrent Vulvovaginal Candidiasis. 64(1656):124.
- Tso G, et al. The Elusive Anti-Candidal Vaccine: Lessions From the Past and Opportunities for the Future. Front Immunol. 2018. 9:897. doi:10.3389/fimmu.2018.00897.
- Sobel, J. D. The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Curr. Infect. Dis. Rep 2006; 8:427–433.
- Sobel, J D. The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Curr. Infect. Dis. Rep 2006; 8:427–433.
- Beigi RH., Meyn LA, Moore DM, et al. Vaginal yeast colonization in non-pregnant women: a longitudinal study. Obstet Gynecol 2004;104:926–30.
- Sobel JD, Faro S, Force R, Foxman B, Ledger W, Nyirjesy P, Reed D, and Summers P. Vulvovaginal candidiasis: epidemiological, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol 1998; 178:203–211.
- Sobel, J. D. The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Curr. Infect. Dis. Rep 2006; 8:427–433.
- Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors, Critical Reviews in Microbiology. 2015.DOI: 10.3109/1040841X.2015.1091805
- Okungbowa FI, Isikhuemhen OS, Dede AP. The distribution frequency of Candida species in the genitourinary tract among symptomatic individuals in Nigerian cities. Rev Iberoam Micol 2003; 20:60–3.
- Andrioli JL, Oliveira GSA, Barreto CS, et al. Frequeˆncia de leveduras em fluido vaginal de mulheres com e sem suspeita clı´nice de candidı´ase vulvovaginal. Rev Bras Ginecol e Obs 2009; 31:300–4
- Bradshaw CS, Morton AN, Garland SM, et al. Higher risk behavioral practices associated with bacterial vaginosis compared with vaginal candidiasis. Obstet Gynecol 2005; 106:105–14.
- Grigoriou O, Baka S, Makrakis E, et al. Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. Eur J Obstet Gynecol Reprod Biol 2006; 126:121–5
- Tibaldi C, Cappello N, Latino MA, et al. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Cain Microbial Infect 2009; 15:670–9
- Ahmad A, Khan AU. Prevalence of Candida species and potential risk factors for vulvovaginal candidiasis in Aligarh, India. Eur J Obstet Gynecol Reprod Biol 2009; 144:68–71.
- Mohanty S, Xess I, Hasan F, et al. Prevalence and susceptibility to fluconazole of Candida species causing vulvovaginitis. Indian JMed Res 2007; 126:216–19.
- Vijaya D, Dhanalakshmi TA, Kulkarni S. Changing trends of vulvovaginal candidiasis. J Lab Physicians 2014; 6:28–30.
- Jafarzadeh L, et al. Vulvovaginal Candidiasis: An overview of mycological, clinical, and immunological aspects. J. Obstet. Gynaecol. Res. 2022; 46 (7):1546-1560.
- Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors, Critical Reviews in Microbiology. 2015.DOI: 10.3109/1040841X.2015.1091805
- Amouri I, Sellami H, Borji N, et al. Epidemiological survey of vulvovaginal candidosis in Sfax, Tunisia. Mycoses. 2011; 54:499–505.
- Mahmoudi RM, Zafarghandi S, Abbasabadi B, Tavallaee M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur J Obstet Gynecol Retrod Biol. 2011;155:199–203.
- Richter SS, Galask RP, Messer SA, et al. Anti fungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbial. 2005; T43:2155–62.
- Amouri I, Sellami H, Borji N, et al. Epidemiological survey of vulvovaginal candidosis in Sfax, Tunisia. Mycoses. 2011; 54:499–505.
- Mahmoudi RM, Zafarghandi S, Abbasabadi B, Tavallaee M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur J Obstet Gynecol Retrod Biol. 2011;155:199–203.
- Richter SS, Galask RP, Messer SA, et al. Anti-fungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbial. 2005; 43:2155–62.
- Linhares IM., Witkin SS, Miranda SD, et al. Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by culture. Infect Dis Obstet Gynecol 2001; 9:221-5.
- Lynch ME, Sobel JD. Comparative in vitro activity of antimycotic agents against pathogenic vaginal yeast isolates. J.Med Vet Mycol 1994; 32:267–74
- Sobel JD, Leamna D. Suppressive maintenance therapy of recurrent bacterial vaginosis utilizing 0.75% metronidazole vaginal gel. In: Abstracts of the second international meeting on bacterial vaginosis. Aspen, 1998
- Spinillo A, Capuzzo E, Gulminetti R, et al. (1997b). Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 176:138–41
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;396:1961-1971.
- Zangl I, et al. The role of Lactobacillus species in the control of Candida via biotrophic interactions. Microbial Cell, January 2020;7(1). Open Access: www.microbialcell.com
- Ferris, DG, Nyirjesy, P, Sobel, JD, et al. Over-the-counter anti-fungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002; 99:419.
- Richter SS, Galask RP, Messer SA, et al. Anti fungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbial. 2005; 43:2155–62.
- Nyerjesy P, Weitz M, Grody M, Lorber B. Over-the-counter and alternative medicines in the treatment of chronic vaginal symptoms. Obstet Gynecol 1997; 90:50–3.
- Dan M, Segal R, Marder V, Leibovitz A. Candida colonization of the vagina in elderly residents of a long-term-care hospital. Eur Clin Microbiol Infect Dis 2006; 25:394–6
- Fan SR, Liu XP, Li JW. Clinical characteristics of vulvovaginal candidiasis and antifungal susceptibilities of Candida species isolates among patients in southern China from 2003 to 2006. J Obstet Gynaecol Res 2008b;34:561–66
- Goswami R, Dadhwal V, Tejaswi S, et al. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic status. J Infect 2000; 41:162–6
- Corsello S, Spinillo A, Osnengo G, et al. An epidemiological survey of vulvovaginal candidiasis in Italy. Eur J Obstet Gynecol Reprod Biol 2003; 110:66–72
- Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors, Critical Reviews in Microbiology. 2015.DOI: 10.3109/1040841X.2015.1091805
- Makanjoula O, et al. An Update on the Roles of Non-Albicans Candida Species in Vulvovaginitis. J. Fungi 2018; 4(121); doi 10.3390/jof4040121.
- Tso G, et al.The Elusive Anti-Candida Vaccine: Lessons From the Past and Opportunities for the Future. Front Immunol. 9:897. doi: 10.3389/fimmu.2018.00897.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;396:1961-1971.
- Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol 2003; 189:1297–300
- Richter SS, Galask RP, Messer SA, et al. Anti fungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbial. 2005; 43:2155–62
- Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract; 1983; 16:919–24
- Sobel, JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985; 152:924.
- Lacour M, Zunder T, Huber R, Sander A, Daschner F. Frank U. The pathogenetic significance of intestinal Candida colonization: a systematic review from an interdisciplinary and environmental medical point of view. Int J Hyg Environ Health. 2002;205:257-268.
- Reed B, Zazove P, Pierson D, et al. Candida transmission and sexual behaviors as risks for a repeat episode of Candida vulvovaginitis. J Womens Health (Larchmt.) 2003; 112:979-89
- Sobel JD. Vulvovaginal candidiasis. In: Holmes KK, Mardh P-A, Sparling PF et al, eds. Sexually Transmitted Diseases. New York: McGraw-Hill, 1990.
- Makanjoula O, et al. An Update on the Roles of Non-albicans Candida Species in Vulvovaginitis. J. Fungi. 2018, 4(121); doi:10.3390/jof4040121.
- Wira C, Grant-Tschudy K, Crane-Goudreau M. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immune 2005; 53:65–76
- Wira C, Fahey J, Stentman C, et al. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev; 2005 206:303–353
- Calderone RA, Gow NA. Host recognition by Candida species. In: Candida and candidiasis, Calderone RA (Ed), ASM Press, Washington, DC. 2002. p.67
- Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors, Critical Reviews in Microbiology. 2015.DOI: 10.3109/1040841X.2015.1091805
- Cassone A, De Bernardis F, Santoni G. Anticandidal immunity and vaginitis: Novel opportunities for immune intervention. Infect Immune 2007; 75(10):4675-86.
- Tso GWH, et al. The Elusive Anti-Candida Vaccine: Lessons From the Past and Opportunities for the Future. Front. Immunol. 9:897. doi:10.3389/fimmu.2018.00897.
- Powell BL. Identification of a 173-estradiol-binding protein in Candida albicans and Candida glabrata. Exp Mycology. 1984;8:304.
- Achkar J, Fries B. Candida infections of the genitourinary tract. Clin Microbial Rev 2010; 23(3):253-73
- Naglik J, Challacombe S, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbial Mol Biol Rev 2003; 67:400
- Zangl I, et al. The role of Lactobacillus species in the control of Candida via biotrophic interactions. 2020 Microbial Cell; 7(1). doi:10.15698/mic2020.01.702.
- Jafarzadeh L, Ranjbar M, Nazari T, Naeimi Eshkaleti M, Aghaei Gharehbolagh S, Sobel JD, Mahmoudi S. Vulvovaginal candidiasis: An overview of mycological, clinical, and immunological aspects. J Obstet Gynaecol Res. 2022 Jul;48(7):1546-1560. doi: 10.1111/jog.15267. Epub 2022 Apr 20. PMID: 35445492.
- Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992; 14:S148–S153
- Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis 1992; 14:S148–S153
- Ringdahl E. Recurrent vulvovaginal candidiasis. Mo Med 2006; 103: 165–168
- Sobel JD, Wiesenfield H, Martens M, Danna P, et al. Maintenance therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–883
- BautersT, Dhont M, Temmerman M, Nelis H. Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am J Obstet Gynecol 2002;187:569–574
- Jackson S, Mullings A, Rainford L, Miller A. The epidemiology of mycotic vulvovaginitis and the use of anti-fungal agents in suspected mycotic vulvovaginitis and its implications for clinical practice. West Indian Med 2005; 54:192-5
- Richter SS, Galask RP, Messer SA, et al. Anti fungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbial. 2005; 43:2155–62.
- Ventolini G., Baggish M, Walsh P. Vulvovaginal candidiasis from non-albicans species: retrospective study of recurrence rate after fluconazole therapy. J Reprod Med 2006;51:475–478
- Tso G, et al. The Elusive Candida Vaccine: Lessons from the Past and Opportunities for the Future. Frontiers in Immunology, April 2018, Vol 9, article 897. doi:10,3389/fimmu.2018.00897.
- Makanjoula O, et al. An Update on the Roles of Non-albicans Candida species in Vulvovaginitis. J. Fungi 2018;4(121); doi:10.3390/jof4040121.
- Vazquez J, Sobel J, Odds F, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Disease 1994; 170:1566
- Tasic S, Tasic N, Tasic A, Mitrovic A. Recurrent genital candidosis of women; consequence of reinfection or relapse. Med Biol. 2002;9:217-222.
- Witkin S. S. Immunology of the vagina. Clin Obstet Gynecol 1993; 36:122–128
- Fidel P. History and update on host defense against vaginal candidiasis. Am J Reprod Immunol 2007; 57:2-12
- Babula O, Lazdane G, Kroicka J, Ledger W, and Witkin S. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin Infect Dis 2003 ;37:733–737
- Babula, Lazdane G, Kroicka J, et al. Frequency of interleukin-4 (IL-4) 589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin Infect Dis 2005; 40: 1258–1262
- Cassone A, De Bernardis F, Santoni G. Anticandidal immunity and vaginitis: novel opportunities for immune intervention. Infect Immun 2007; 75:4675-869
- Sahu S, et al. Vaccines against Candidiasis: Status, challenges, and emerging opportunity. Front. Cell. Infect. Microbiol. 12:1002406. doi: 10.3389/fcimb.2022.1002406.
- Richardson M, Rautemaa R. How the host fights against Candida infections. Front Biosci 2009; 14:4363–75
- Cole A. Innate host defense of human vaginal and cervical mucosal. Curr Top Microbial Immunol 2006; 306:199-230
- Fidel P. History and update on host defense against vaginal candidiasis. Am J Reprod Immunol 2007; 57:2-12
- Pudney J, Quayle A, Anderson D. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 2005; 73:1253-63
- Cassone A, De Bernardis F, Santoni G. Anticandidal immunity and vaginitis: novel opportunities for immune intervention. Infect Immun 2007; 75:4675-869
- Rolides E, Holmes A, Blake C, et al. Effects of granulocyte colony-stimulating factor and interferon-gamma on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J Leukoc Biol 1995; 57:651-6
- Gavira J, van Burik J, Dale D, et al. Modulation of neutrophil-mediated activity against the pseudohyphal form of Candida albicans by granulocyte colony-stimulating factor (G-Csf) administered in vivo. J Infect Dis 1999; 179:1301-4
- Willems HME, et al. Comparative Analysis of the Capability of the Candida Species to Elicit Vaginal Immunopathology. Infect Immun. 2018. 86:e00527-18. https://doi.org/10.1128/IAI.00527-18.
- Martinez J, Gil M, Lopez-Ribot J, Chaffin W. Serologic response to cell wall mannoproteins and proteins of Candida albicans. Clin Microbiol Rev 1998; 11:121-41
- Casadevall A, Cassone A. Bistoni F, et al. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med Mycol 1998; 36 Suppl 1:95-105
- Kalia N, et al. Ann Clin Microb Antimicrob 2020.19:5. https://doi.org/10.1186/s12941-020-0347-4.
- Zhang M, Kozel T. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect Immun 1998; 66:4845-50
- Spellberg B, Ibrahim A, Avenissian V, et al. The anti-Candida albicans vaccine composed of recombinant N terminus of Als 1 p reduces fungal burden and improves survival in both immuocompetent and immunocompromised mice. Infec Immun 2005; 73:6191-93
- Cassone A, De Bernardis F, Santoni G. Anticandidal immunity and vaginitis: novel opportunities for immune intervention. Infect Immune 2007; 75:4675-86
- Eckert L, Hawes S, Stevens C. Vulvovaginal candidiasis, clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998; 92:757-765
- Cotch M, Hillier S, Eschenbach D. epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity study Group. Am J Obstet Gynecol 1998; 178:374-380
- Powell BL. Identification of a 17beta-estradiol-binding protein in Candida albicans and Candida glabrata. Exp Mycology. 1984;8:304.
- Goswami R, Dadhwal V. Tejaswi S, et al. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycemic status. J Infect. 2000;41:162-166.
- Donders GG, Prenen H, Verbeke G, Reybrouck R. Impaired tolerance for glucose in women with recurrent vaginal candidiasis. Am J Obstet Gynecol. 2002;187:989-993.
- Sobel JD and Mitchell C. Candida vulvovaginitis: Clinical manifestations and diagnosis. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com. (Accessed 12/8/2022).
- Bluestein D, Rutledge C, Lumsden L. Predicting the occurrence of antibiotic-induced candidal vaginitis (AICV). Fam Prac Res J. 1991;11:319-326.
- Pultz NJ, Stiefel U, Ghannoum M, Helfand Ms. Donskey, CJ. Effect of parenteral antibiotic administration on the establishment of intestinal colonization by Candida glabrata in adult mice. Antimicrob Agents Chemother. 2005;49:438-440.
- Auger P, Joly J. Microbial flora associated with Candida albicans vulvovaginitis. Obstet Gynecol. 1980;55:397-401.
- Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing post-antibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ. 2004; 329:548.
- Sobel, JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985; 152:924.
- Merenstein D, Hu H, Wang C, et al. Colonization by Candida Species of the Oral and Vaginal Mucosa in HIV-Infected and Noninflected Women. AIDS Res Hum Retroviruses. 2013 Jan;29(1): 30–34
- Sheary B, Dayan L. Clinical practice. Recurrent vulvovaginal candidiasis. Aust Fam Physician. 2005;34:147-150.
- Duerr A, Helig C, Meikle S, et al. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus-infected women: Risk factors and severity. Obstet Gynecol 2003; 101:548-56.
- Makanjoula O, et al. An Update on the Roles of Non-albicans Candida Species in Vulvovaginitis. J. Fungi 2018. 4,121; doi:10.3390/jof4040121.
- Barbone f, Austin H, Louv WC, Alexander WJ. A follow-up study of methods of contraception, sexual activity, and rates of trichomoniasis, candidiasis, and bacterial vaginosis. Am J Obstet Gynecol.1990;163:510-514.
- Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health. 1990;80:329-331.
- Sobel JD and Mitchell C. Candida vulvovaginitis: Clinical manifestations and diagnosis. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA:UpToDate Inc. http://www.uptodate.com (Accessed 12/8/2022)
- Foxman B, Marsh JV, Gillespie B, Sobel JD. Frequency and response to vaginal symptoms among white and African American women: results of a random digit dialing survey. J Womens Health. 1998;7:1167-1174.
- Schmid J, Rotman M, Reed B, Pierson CL, Soll Dr. Genetic similarity of Candida albicans strains from vaginitis patients and their partners. J Clin Microbiol. 1993;31-39.
- Reed BD. Risk factors for Candida vulvovaginitis. Obstetric Gynecol Survey. 1992;47:551.
- Schmid J, Rotman M, Reed B, Pierson CL, Soll DR. Genetic similarity of Candida albicans strains from vaginitis patients and their partners. J Clin Microbiol. 1993;31-39.
- Reed BD, Zazove P, Pierson CL, Gorenflo Dw, Horrocks J. Candida transmission and sexual behaviors are risks for a repeat episode of Candida vulvovaginitis. J Womens Health. (Larchmont) 2003;12:979-989.
- Bradshaw CS, Morton AN, Garland SM, Morris MB, Moss LM, Fairley CK. Higher-risk behavioral practices associated with bacterial vaginosis compared with vaginal candidiasis. Obstet Gynecol. 2005;106:105-114.
- Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203-211.
- Sobel JD and Mitchell C. Candida vulvovaginitis: Clinical manifestations and diagnosis. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com. (Accessed 12/8/2022).
- Neves NA, Carvalho LP, De Oliviera MA, et al. Association between atopy and recurrent vaginal candidiasis. Clin Exp Immunol. 2005;142:167-171.
- Powell BL. Identification of a 17beta-estradiol-binding protein in Candida albicans and Candida glabrata. Exp Mycology. 1984;8:30.
- Chatzivasileiou P and Vyzantiadis T. Vaginal yeast colonisation: From a potential harmless condition to clinical implications and management approaches – A literature review. Mycoses 2019;62:638-650. doi:10.1111/myc.12920.
- Nyrijesy P, Alexander AB, Weitz MV. Vaginal Candida parapsilosis: pathogen or bystander? Infect Dis Obstet Gynecol. 2005;13:37-41.
- Sobel JD, Chaim W. Treatment of Torulopsis glabrata vaginitis: A retrospective review of boric acid therapy. Clin Infect Dis. 1997; 24:649.
- Schwebke JR, Gaydos CA, Nyirjesy P, Paradis S, Kodsi S, Cooper CK. Diagnostic Performance of a Molecular Test versus Clinician Assessment of Vaginitis. J Clin Microbiol. 2018 May 25;56(6):e00252-18. doi: 10.1128/JCM.00252-18. PMID: 29643195; PMCID: PMC5971525.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;396:1961-1971.
- Sobel JD. Candida vulvovaginitis. In: UpToDate. Basow DE (Ed). UpToDate, Waltham, MA, 2016.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;396:1961-1971.
- Ferris, DG, Nyirjesy, P, Sobel, JD, et al. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002; 99:419.
- Sobel JD and Mitchell C. Candida vulvovaginitis: Clinical manifestations and diagnosis. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com. (Accessed 12/8/2022).
- Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, Reed BD, Summers P. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203.
- Pappas P, Kauffman C, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503-535
- Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, Reed BD, Summers P. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203.
- Sobel JD. Candida vulvovaginitis. In: UpToDate. Basow DE (Ed). UpToDate, Waltham, MA, 2015.
- Sobel JD. Candida vulvovaginitis. In: UpToDate. Basow DE (Ed). UpToDate, Waltham, MA, 2016.
- Center for Disease control and Prevention, June, 2015 www.cdc.gov/fungal/diseases/candidiasis/index.html
- Sobel JD. Candida vulvovaginitis: Treatment. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com. (Accessed 12/8/2022).
Ibrexafungerp is a new, first-in-class triterpenoid antifungal for use in females with uncomplicated vulvovaginal candidiasis. The mechanism of action as a glucan synthase inhibitor is inhibition of fungal cell wall formation, and it is the first antifungal for treatment of vaginal candidiasis which is fungicidal. Since it is currently substantially more expensive than other candida treatments, its optimal treatment niche may be for patients who are allergic to fluconazole or other triazoles, do not tolerate fluconazole or other triazoles, and/or have candida infections which are resistant to fluconazole. The usual dosing is two 150 mg tablets (300 mg) taken twice, 12 hours apart (600 mg total). Individuals taking strong CYP3A inhibitors should reduce the dose to 150 mg twice daily for 2 doses. (CYP inhibitors include glucocorticoids, rifampin, phenytoin, carbamazepine, phenobarbital, grapefruit juice, erythromycin, ketoconazole, clarithromycin, and verapamil). Use of Ibrexafungerp is contraindicated in pregnancy and lactation due to lack of human data and evidence of fetal harm in animal studies. Potential adverse effects include diarrhea, nausea, abdominal pain, dizziness and vomiting. The cost for one course of treatment in 2021 was $475.179Ibrexafungerp (Brexafemme) for Vulvovaginal Candidiasis. The Medical Letter on Drugs and Therapeutics. Sept 6, 2021; 63(1632):141-143.
- Pappas, PG, Kauffman, CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48:503.
- Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: Comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol. 2001; 185:363.
- Sobel JD. Candida vulvovaginitis: Treatment. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA:UpToDate Inc. http://www.uptodate.com. (Accessed 12/8/2022)
- Oteseconazole (Vivjoa) for Recurrent Vulvovaginal Candidisis. The Medical Letter on Drugs and Therapeutics. Aug 8, 2022;64(1656):124-126.
- Sobel JD. Candida Vulovovaginitis: Treatment. Barbieri RL and Kauffman CA, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed 12/8/2022).
- Sobel JD. Management of patients with recurrent vulvovaginal candidiasis. Drugs. 2003;63:1059-1066.
- Sood G, Nyirjesy P, Weitz MV, Chatwani A. Terconazole cream for non-Candida albicans fungal vaginitis: results of retrospective analysis. Infec Dis Obstet Gynecol. 2000; 8(5-6):240-243.
- Sobel JD, Chaim W. Treatment of Torulopsis glabrata vaginitis: A retrospective review of boric acid therapy. Clin Infect Dis. 1997; 24:649.
- Iavazzo C, Gkegkes I, Zarkada I, Falagas M. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence J Womens Health (Larchmt) 2011; 20:1245-55
- Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol. 2003; 189:1297.
- Center for Disease control and Prevention, June, 2015 www.cdc.gov/fungal/diseases/candidiasis/index.html.
- Lopez-Rangel E, Van Allen MI. Prenatal exposure to fluconazole: an identifiable dysmorphic phenotype. Birth Defects Res A Clin Mol Teratol. 2005;73:919-923.
- Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
- http://www.fda.gov/Drugs/DrugSafety/ucm266030.htm
- Zhu Y, et al. Oral fluconazole use in the first trimester and the risk of congenital malformations: a population based cohort study. BMJ 2020;369:m1494.
- Budani MC, et al. Maternal use of fluconazole and congenital malformations in the progeny: a meta-analysis of the literature. Reprod. Toxicol. 2021, Mar;100:42-51. doi10.1016/j.reprotox.2020.12.018.
- Geller ML, Nelson AL. Bacterial vaginosis and vulvovaginal candidiasis. Women’s Health Prim Pract. 2003; 6:147-150.
- Carcinogenic Potency Database (CPDB)
- extoxnet.orst.edu
- http://www.llli.org/docs/482.pdf
- Drinkwater, P. Gentian Violet — Is It Safe? Australian N Z J Obstet Gynaecol. 1990; 30: 65-69.
- Bradshaw CS, Morton AN, Garland SM, Morris MB, Moss LM, Fairley CK. Higher-risk behavior practices associated with bacterial vaginosis compared with vaginal candidiasis. Obstet Gynecol. 2005;106:105-114.
- Center for Disease Control STI Guidelines 2021.https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Peebles K, Velloza J, et al. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex Transm Dis. 2019; 46(5):304.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505–23.
- Peebles K, Velloza J, et al. High Global Burden and Costs of Bacterial Vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46(5): 304.
- Peebles K, Velloza J, et al. High Global Burden and Costs of Bacterial Vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46(5): 304.
- Peebles K, Velloza J, et al. High Global Burden and Costs of Bacterial Vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46(5): 304.
- Ledger WJ, Witkin SS. Vulvovaginal Infections. Washington, D.C., ASM Press, 2007: 9.
- Onderdonk AB, Delaney ML, Fichhorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev 2016. 29:223-238
- Marrazo J. Evolving issues in understanding and treating bacterial vaginosis. Expert Rev Anti Infect Ther. 2004; 2:913-922
- Ravel J, Gajer P, Abdo Z, Schneider GM, et al. Vaginal microbiome of reproductive age women. Proc Natl Acad Sci USA. 2011 Mar 15;108 (Suppl 1): 4680-4687.
- Fredericks D, Fiedler T, Marazzo J. Molecular identification of bacteria associated with bacterial vaginosis. NEJM. 2005; 353:1899-1911.
- Muzny CA, Łaniewski P, Schwebke JR, Herbst-Kralovetz MM. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr Opin Infect Dis. 2020 Feb;33(1):59-65.
- Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis, and treatment. Curr Opin Infect Dis 2013; 26:86.
- Machado D, Castro J, Palmeira-de-Oliveira A, et al. Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions. Front Microbiol. 2016 Jan 20; 6:1528.
- Alvesa P, Castroa J, Sousa C, et al. Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using an in vitro biofilm formation model. J Infect Dis. 2014 Aug 15; 210(4);593-6. doi: 10.1093/infdis/jiu131. Epub 2014 Mar 4.
- Hay P. Bacterial vaginosis. F1000Res. 2017 Sep 27;6:1761. doi: 10.12688/f1000research.11417.1. PMID: 29043070; PMCID: PMC5621139.
- Livengood CH. Bacterial vaginosis: an overview for 2009. Rev Obstet Gynecol. 2009;2:28-37.
- Rymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013 May 15;207(10):1491-1497.
- Redelinghuys MJ, Geldenhuys J, Jung H, Kock MM. Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities. Front Cell Infect Microbiol. 2020 Aug 11;10:354. doi: 10.3389/fcimb.2020.00354. PMID: 32850469; PMCID: PMC7431474.
- Nunn K, Wang Y, Lai S. Enhanced Trapping of HIV-1- by Human Cervicovaginal Mucus is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio. 2015; 6(5): e01084-15.
- Wright R, Fettweis J, Eaves L et al. Vaginal microbiome Lactobacillus crispatus is heritable among European American women. Commun Biol. 2021; 4:872.
- Klebanoff, MA, Nansel, TR, Brotman, RM, Zhang, J, Yu, KF, et al. Personal Hygienic Behaviors and Bacterial Vaginosis. Sex Transm Dis. 2010 Feb;37(2):94-99.
- Morris M, Nicoll A, Simms I, et al. Bacterial vaginosis: a public health review. BJOG. 2001; 108:439-50.
- Manhart LE, Khosropour CM, Liu C, et al.: Bacterial vaginosis-associated bacteria in men: association of Leptotrichia/Sneathia spp. with nongonococcal urethritis. Sex Transm Dis. 2013;40(12):944–9. 10.1097/OLQ.0000000000000054
- Center for Disease Control STI Treatment Guidelines 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Fethers KA, Fairley CK, Morton A, et al. Early sexual experiences and risk factors for bacterial vaginosis. J Infect Dis. 2009; 200:1662.
- Verstraelen H, Verheist R, Vaneechoutte M, Temmerman M. The Epidemiology of Bacteria Vaginosis in Relation to Sexual Behaviour. BMC Infectious Disease. 2010 10;81.
- Fethers KA, Fairley CK, Morton A, et al. Early sexual experiences and risk factors for bacterial vaginosis. J Infect Dis. 2009; 200:1662.
- Vodstrcil LA, Walker SM, Hocking JS, et al.: Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042–53. 10.1093/cid/ciu1130
- Bradshaw CS, Walker SM, Vodstrcil LA, et al. The influence of behaviors and relationships on the vaginal microbiota of women and their female partners: the WOW Health Study. J Infect Dis 2014; 209:1562.
- Bilardi J, Walker S, Mooney-Somers J, Temple-Smith M, et al. Women’s views and experiences of triggers for onset of bacterial vaginosis and exacerbating factors associated with recurrence. PLoSOne 2016 Mar 1; 11(3) e0150272.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis 2013; 56:777
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Esber A, Vicetti Miguel RD, Cherpes TL, et al. Risk of bacterial vaginosis among women with herpes simplex virus type 2 infection: a systematic review and meta-analysis. J Infect Dis 2015; 212:8
- Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infectious Dis 2010 Dec 15; 202(12): 1907-15.
- Gallo MF, Macaluso M, Warner L, et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemio 2012 Mar; 22(3): 213-20.
- MyerL, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis 2005; 192:1372
- Jamieson DJ, Duerr A, Klein RS, et al. Longitudinal analysis of bacterial vaginosis: findings from the HIV epidemiology research study. Obstet Gynecol 2001; 98:656
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Lallar M, Nanda S, Nandar R. Lower genital tract infections in HIV positive women: can we afford to miss? J Obstet Gynaecol India. 2015 Feb; 65(1): 45–49.
- Hay P. Bacterial Vaginosis. Medicine 2014;42(7):359-63.
- Sobel J and Mitchell C. Bacterial vaginosis: Clinical manifestations and diagnosis. UpToDate.com, 2022
- Wiensenfeld HC, Macio I. The infrequent use of office-based diagnostic tests for vaginitis. Am J Obstet Gynecol. 1999;18:39-41.
- Hillier SL, Austin M, et al. Diagnosis and Treatment of Vaginal Discharge Syndromes in Community Practice Settings. Clin Infect Dis. 2021;72(9):1538.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74:14.
- Center for Disease Control STI treatment guidelines, 202. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Eschenbach DA, Hillier SL, Critchlow C, et al. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988; 158:819.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991; 29:297.
- Sobel JD. Bacterial vaginosis. UpToDate.com 2022.
- Camus C, Penaranda G, Khiri H, et al. Acceptability and efficacy of vaginal self-sampling for genital infection and bacterial vaginosis: A cross-sectional study. PLoS One. 2021 Nov 18;16(11):e0260021.doi:10.1371/journal.pone.0260021. PMID:34793548;PMCID:PMC8601421.
- Camus C, Penaranda G, Khiri H, et al. Acceptability and efficacy of vaginal self-sampling for genital infection and bacterial vaginosis: A cross-sectional study. PLoS One. 2021 Nov 18;16(11):e0260021.doi:10.1371/journal.pone.0260021. PMID:34793548;PMCID:PMC8601421.
- Greene JF, 3rd, Kuehl TJ, Allen SR. The Papanicolaou smear: inadequate screening test for bacterial vaginosis during pregnancy. Am J Obstet Gynecol. 2000; 182:1048.
- Andrews WW, Hauth JC, Cliver SP, et al. Association of asymptomatic bacterial vaginosis with endometrial microbial colonization and plasma cell endometritis in nonpregnant women. Am J Obstet Gynecol. 2006; 195:1611.
- MacDermott RI. Bacterial vaginosis. Br J Obstet Gynaecol.1995; 102:92.
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Myer, L, Denny, L, Telerant, R, et al. Bacterial Vaginosis and Susceptibility to HIV Infection in South African Women: A Nested Case-Control Study. J Infect Dis. 2005; 192:1372.
- Myer, L, Denny, L, Telerant, R, et al. Bacterial Vaginosis and Susceptibility to HIV Infection in South African Women: A Nested Case-Control Study. J Infect Dis. 2005; 192:1372.
- Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005; 162:585.
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Cauci S, Guaschino S, DeAloysio D, Driussi S, De Santo D, Penacchioni P, et al. Interrelationships of interleukin-8 with interleukin- 1 beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9:53-58.
- Hauth JC, MacPherson C, Carey C, Klebanoff MA, Hillier SL, Ernest JM, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Early pregnancy threshold vaginal pH and gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am J Obstet Gynecol. 2003;188:831-835.
- Culhane JF, Nyirjesy P, McCollum K, Goldenberg R, Gelber S, Cauci S. Variation in vaginal immune parameters and microbial hydrolytic enzymes in bacterial vaginosis positive pregnancy women with and without Mobiluncus species. Am J Obstet Gynecol. 2006; 195:516-521.
- Cauci S, Guaschino S, DeAloysio D, Driussi S, De Santo D, Penacchioni P, et al. Interrelationships of interleukin-8 with interleukin- 1 beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9:53-58.
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Center for Disease Control STI Treatment Guidelines 2021 https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Soper DE. Bacterial vaginosis and surgical site infections. SOAm J Obstet Gynecol. 2020;222(3):219. Epub 2019 Sep 6.
- Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases Am J Obstet Gynecol, 2007;196(6):e1-6.
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Livengood CH III, Soper DE, Sheehan KL, et al. Comparison of once-daily and twice-daily dosing of 0.75% metronidazole gel in the treatment of bacterial vaginosis. Sex Transmit Dis. 1999;26:137–142.
- Hanson, JM, McGregor, JA, Hillier, SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med. 2000; 45:889.
- Hanson, JM, McGregor, JA, Hillier, SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med. 2000; 45:889.
- UpToDate.com, 2022. Metronidazole drug information
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Sobel J, Peipert JF, McGregor JA, et al. Efficacy of clindamycin vaginal ovule (3-day treatment) vs. clindamycin vaginal cream (7-day treatment) in bacterial vaginosis. Infect Dis Obstet Gynecol. 2001;9:9–15.
- https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2020/01/vaginitis-in-nonpregnant-patients
- Beigi, RH, Austin, MN, Meyn, LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004; 191:1124.
- Nyirjesy P, McIntosh MJ, Gattermeir DJ, Schumacher RJ, Steinmetz JI, Joffrion JL. The effects of intravaginal clindamycin and metronidazole therapy on vaginal lactobacilli patients with bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1277-1282.
- Helms DJ, Mosure DJ, Secor WE, et al. Management of trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol 2008; 198e371-7.
- Tinidazole (Tindamax)–a new option for treatment of bacterial vaginosis. Med Lett Drugs Ther. 2007; 49:73.
- Livengood CH, 3rd, Ferris, DG, Wiesenfeld, HC, et al. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2007; 110:302.
- Ekgren J, Norling BK, Degre M, Midtvedt T. Comparison of tinidazole given as a single dose and on 2 consecutive days for the treatment of nonspecific bacterial vaginosis. Gynecol Obstet Invest. 1988; 26:313.
- Aziz M, Sharifipour D, Abedi P et al. Secnidazole for treatment of bacterial vaginosis: a systematic review. BMC Women’s Health 2019. (19) 121.
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm[/efn_note]
· Metronidazole 500 mg orally twice daily for seven days, or · Metronidazole 250 mg orally three times daily for seven days, or · Clindamycin 300 mg orally twice a day for seven days The Centers for Disease Control and Prevention no longer discourage the use of metronidazole in the first trimester. At this time, the CDC states that the regimens recommended for non-pregnant women can also be used in pregnancy (except for tinidazole and secnidazole).
Treatment of asymptomatic BV among pregnant women at high risk for preterm delivery (i.e. those with a previous preterm birth or late miscarriage) has been evaluated by multiple studies with mixed results. At this time, the data do not support screening or treatment, but further studies are underway. 286Sobel JD. Bacterial Vaginosis: Treatment. UpToDate.com; 2022.
- Sobel, JD. Bacterial Vaginosis: Treatment; UpToDate.com 2022.
- Metronidazole in Drugs and Lactation Database. https://www.ncbi.nlm.nih.gov/books/NBK501315/, last update March 17, 2021
- American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001; 108:776.
- Sobel JD. Bacterial Vaginosis: Treatment. UpToDate.com 2022
- Coudray MS, Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020 Feb;245:143-148. doi: 10.1016/j.ejogrb.2019.12.035. Epub 2019 Dec 24. PMID: 31901667; PMCID: PMC6989391
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- ACOG Practice Bulletin No. 72. Clinical management guidelines for obstetrician-gynecologists: Vaginitis. Obstet Gynecol. 2006;107:1195016.
- Verwijs MC, Agaba SK, Darby AC, van de Wijgert. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. JHHM SOAm J Obstet Gynecol. 2020;222(2):157.e1. Epub 2019 Aug 9.
- Surapaneni S, Akins R, and Sobel JD Recurrent Bacterial Vaginosis: an unmet therapeutic challenge. Experience with a combination pharmacotherapy long-term suppressive regimen. 2021. Sexually transmitted diseases; 48(10, 761-765. https://doi.org/10.1097/OLQ.0000000000001420
- Surapaneni S, Akins R, and Sobel JD Recurrent Bacterial Vaginosis: an unmet therapeutic challenge. Experience with a combination pharmacotherapy long-term suppressive regimen. 2021. Sexually transmitted diseases; 48(10, 761-765. https://doi.org/10.1097/OLQ.0000000000001420
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. AJ OB GYN. 2009. 200(1),42.e1-42.e427.
- Center for Disease Control, STI Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/bv.htm
- Vodstril LA, Muzny CA, Plummer EL, Sobel JD, and Bradshaw CS. Bacterial vaginosis: drivers of recurrence and challenges and opportunities in partner treatment. BMC Medicine. 2021. 19(1), 194
- Vodstril LA, Muzny CA, Plummer EL, Sobel JD, and Bradshaw CS. Bacterial vaginosis: drivers of recurrence and challenges and opportunities in partner treatment. BMC Medicine. 2021. 19(1), 194
- Sobel J. Bacterial Vaginosis. UptoDate. 2022
- Bradshaw, CS, Morton, AN, Hocking, J, et al. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence. J Infect Dis. 2006; 193:1478.
- Sanchez S, Garcia PJ, Thomas KK, et al. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol 2004; 191:1898.
- Ness RB, Hillier SL, Richter HE, Soper DE, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstetrics and Gynecology. 2002. 100(4), 765.
- Joseph RJ, Ser HL, Kuai YNH, et al. Finding a balance in the vaginal microbiome: how do we treat and prevent the occurrence of bacterial vaginosis? Antibiotics (Basal, Switzerland) 2021. 10(6), 719
- Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S,k et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N Engl J Med. 2020;382(20):1906
- US Food and Drug Administration. MedWatch safety alerts for human medical products. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/.
- Lev-Sagie A, Goldman-Wohl D, Cohen Y, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25(10):1500. Epub 2019 Oct 7.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019 Sep 20;8:F1000 Faculty Rev-1666. doi: 10.12688/f1000research.19972.1. PMID: 31583080; PMCID: PMC6758837.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004; 291:1368.
- Centers for Disease Control Sexually Transmitted Disease Guidelines, July, 2021. https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Menezes CB, Frasson AP, Tasca T. Trichomoniasis – are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb Cell. 2016 Jun 27;3(9):404-419. doi: 10.15698/mic2016.09.526. PMID: 28357378; PMCID: PMC5354568.
- CDC Statistics: https://www.cdc.gov/std/trichomonas/stats.htm#:~:text=Prevalence&text=The%20prevalence%20of%20Trichomonas%20vaginalis,participated%20in%20NHANES%202013%2D2016.
- Flagg EW, Meites E, Phillips C, Papp J, Torrone EA. Prevalence of Trichomonas vaginalis Among Civilian, Noninstitutionalized Male and Female Population Aged 14 to 59 Years: United States, 2013 to 2016. Sex Transm Dis. 2019 Oct;46(10):e93-e96.
- Huppert, J. Trichomonas in teens: an update. Curr Opinion Obstet Gynecol. 2009; 21(5):372-378.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317
- Centers for Disease Control Sexually Transmitted Disease Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com, 2022.
- Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin Infect Dis. 2007; 45:1319.
- Huppert, J. Trichomonas in teens: an update. Curr Opinion Obstet Gynecol. 2009; 21(5):372-378.
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com, 2022
- Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006; 33:747-752.
- Gottlieb SL, Douglas JM Jr, Foster M, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted diseases. J Infect Dis. 2004; 190; 1059-1067.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. I Infect Dis. 2007; 195:698-702.
- Shew ML, Fortenberry JD, Tu W, et al. Association of condom use, sexual behaviors and sexually transmitted infections with the duration of genital human papilloma virus infection among adolescent women. Arch Pediatr Adolesc Med. 2006; 160:151-156.
- Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. 1994; 23:682.
- Cotch MF, Pastorek JG 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997; 24:353.
- Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001; 345:487.
- Centers for Disease Control and Prevention, Sexually Transmitted Disease Guidelines, July, 2021 https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com. 2022
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019 Sep 20;8:F1000 Faculty Rev-1666. doi: 10.12688/f1000research.19972.1. PMID: 31583080; PMCID: PMC6758837.
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep 2021;70(No. RR-4):1–187. DOI: http://dx.doi.org/10.15585/mmwr.rr7004a1external icon.
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com, 2022.
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com. 2022.
- Lowe NK, Neal JL, Ryan-Wenger NA. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol. 2009; 113:89-95.
- Huppert JS, Mortensen JE, Reed JL, et al. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin Infect Dis. 2007; 45:194.
- Huppert, J. Trichomonas in teens: an update. Curr Opinion Obstet Gynecol. 2009; 21(5):372-378.
- Wiese W, Patel SR, Patel SC, et al. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med. 2000; 108:301.
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com, 2022
- Lara-Torre E, Pinkerton JS. Accuracy of detection of Trichomonas vaginalis organisms on a liquid based Papanicolaou smear. Am J Obstet Gynecol. 2003; 188:354-356.
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com, 2022
- Heine, P, McGregor, JA. Trichomonas vaginalis: a reemerging pathogen. Clin Obstet Gynecol. 1993; 36:137.
- Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004; 17:794.
- Centers for Disease Control and Prevention, Sexually Transmitted Disease Guidelines, July 2021.https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Helms, DJ, Mosure, DJ, Secor WE, Workowski KA. Management of Trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol. 2008; 198:370.
- Centers for Disease control and Prevention, July 2021
- Centers for Disease control and Prevention, Sexually Transmitted Disease Guidelines July, 2021. https://www.cdc.gov/std/treatment-guideline6/trichomoniasis.htm.
- Lossick JG. Treatment of sexually transmitted vaginosis/vaginitis. Rev Infect Dis. 1990; 12 Suppl 6:S665.
- Marrazo M. Barriers to infectious disease care among lesbians. Emerg Infect Dis. 2004; 10(11):1974-1978.
- Sobel JD and Mitchell C. Trichomoniasis. UpToDate.com, 2022
- Centers for Disease Control and Prevention, Sexually Transmitted Disease Guidelines, July 2021.https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Caro-Paton T, Carvajal, A, Martin de, Diego I, et al. Is metronidazole teratogenic? A meta-analysis. Br J Clin Pharmacol. 1997; 44:179.
- Einarson, A, Ho, E, Koren, G. Can we use metronidazole during pregnancy and breastfeeding? Putting an end to the controversy. Can Fam Physician. 2000; 46:1053.
- Passmore CM, McElnay JC, Rainey EA, D’Arcy PF. Metronidazole excretion in human milk and its effect on the suckling neonate. Br J Clin Pharmacol. 1988; 26:45.
- Centers for Disease Control and Prevention Sexually Transmitted Disease Guidelines, July 2021.https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Helms DJ, Mosure DJ, Secor WE, et al. Management of trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol 2008; 198e371-7.
- Centers for Disease Control and Prevention, Sexually Transmitted Disease Guidelines, July 2021. https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Center for Disease control and Prevention, June, 2015 http://www.cdc.gov/std/tg2015/default.htm.
- Schwebke JR, Barrientes FJ. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother. 2006; 50:4209.
- Tonkovic-Capin V, Fleming MG, Kleven-Kranz K, Lund MR. Vulvo-vaginitis and perineal cellulitis due to group A streptococcus in an adult woman. Arch Dermatol. 2005; 141:790–792.
- Sobel JD, Funaro D, Kaplan EL. Recurrent group A streptococcal vulvovaginitis in adult women: family epidemiology. Clin Infect Dis. 2007; 44:e43.
- Mead PB, Winn WC. Vaginal-rectal colonization with group A streptococci in late pregnancy. Infect Dis Obstet Gynecol. 2000; 8:217–9.
- Stricker T, Navratil F, Sennhauser FH. Vulvovaginitis in prepubertal girls. Arch Dis Child. 2003;88:324–326.
- Tonkovid-Capin V, Fleming MG, Kleven-Kranz K, Lund MR. Vulvo-vaginitis and perineal cellulitis due to group A streptococcus in an adult woman. Arch Dermatol. 2005; 141:790–792.
- Factor SH, Levine OS, Harrison LH, et al. Risk factors for pediatric invasive group A streptococcal disease. Emerg Infect Dis. 2005; 11:1062-1066.
- Berkelman RI, Martin D. Graham DR, et al. Streptococcal wound infections caused by a vaginal carrier. JAMA. 1982;247:2680-2682.
- McKee WM, Di Caprio JM, Roberts CE Jr, Sherris JC. Anal carriage as the probable source of a streptococcal epidemic. Lancet. 1966; 2:1007-1009.
- Gryska PF, O’Dea Ae. Postoperative streptococcal wound infection: the anatomy of an epidemic. JAMA. 1970;213:1189-11891.
- http://www.mayoclinic.org/diseases-conditions/toxic-shock-syndrome/basics/definition/con-20021326
- Mead PB, Winn WC. Vaginal-rectal colonization with group A streptococci in late pregnancy. Infect Dis Obstet Gynecol. 2000; 8:217–9.
- Gardner H. Desquamative inflammatory vaginitis: a newly defined entity. Am J Obstet Gynecol. 1968; 102:1102-1105.
- Sobel JD. Desquamative inflammatory vaginitis; a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol. 1994; 171(5):1215-1220.
- Nyrijesy P, Sobel JD. Advances in diagnosing vaginitis: development of a new algorithm. Curr Infect Dis Rep. 2005; 7:458-462.
- Sobel JD. Nontrichomonal purulent vaginitis: clinical approach. Curr Infect Dis Rep. 2000; 2:501-505.
- Jones B, Geary I, Alawattegama B, et al. In vitro and in vivo activity of metronidazole against Gardnerella vaginalis, Bacteroides spp and Mobiluncus spp in bacterial vaginosis. Antimicrov Agents Chemother. 1985; 16:89-97.
- Horowitz B. Recurrent and relapsing vaginitis. In: Vaginitis and Vaginosis. Horowitz B, Mardh, P, eds. New York, Wiley-Liss, 1991:230.
- Cibley L, Cibley L Jr. Cytolytic vaginosis. Am J Obstet Gynecol. 1991;165:1245-1247.
- Yang S, Liu Y, et al. Variation of the vaginal Lactobacillus microbiomes in cytolytic vaginosis, J Low Genital Tract Dis. 2020; 24:417-20.
- Cerikcioglu N, Beksac M. Cytolytic vaginosis: misdiagnosed as candidal vaginitis. Infec Dis Obstet Gynecol. 2004; 12(1):13-16.
- Soares R, Viera-Baptista P, et al. Vaginose citolitica: umm entidade sugdaignosticada que minietiza a candidiases vaginal. Acta Obstetrics e Gynecological Portuguese. 2017; 11:10-12.
- Boris S, Covadonga B. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000; 2:543-6.
- Voytik M, Nyirjesy P. Cytolytic Vaginosis: a critical Appraisal of a Controversial Condition.Current Infections Disease reports 2020; Aug 22:26.
- Portman DJ, Gass ML. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North Americal Menopause Society. Menopause 2014; 21:1063-1068.
- Utian W. Menopause. In: Loue S, Sajatovic M, eds. Encyclopedia of Women’s Health. New York: Kluwer Academic, 2004.
- Graziottin A, Leiblum SR. Biological and psychosocial pathophysiology of female sexual dysfunction during the menopausal transition. J Sex Med. 2005;2:133-145.
- Edwards L, Craig R, Roepe K. Attention to the atrophic vagina in postmenopausal women with chronic vulvovaginal symptoms. Presented at the XXIII World Congress of the International Society for the Study of Vulvovaginal Disease, July 27-29, 2015, New York University, New York City, NY.
- The North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355-369.
- The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020 Sep;27(9):976-992. doi: 10.1097/GME.0000000000001609. PMID: 32852449.
- Portman DJ, Gass ML. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014; 21:1063-1068.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas 2004; 49:292-303.
- Levine KB, Williams RE, Hartmann KE. Vulvovaginal atrophy is stongly associated with female sexual dysfunction among sexually active postmenopausal women. Menopause. 2008; 15:661-666.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009; 6:2133-2142
- Palma F, Volpe A, Villa P. Cagnacci A. Writing group of AGATA study: Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas. 2016; 83:40-44
- Biglia N, Bounous V, D’Alonzo M, Ottino L, et al. Vaginal Atrophy in Breast Cancer Survivors: Attitude and Approaches among Oncologists. Clinical Breast Cancer. 2017; 8:611-617
- Pandit, L, Ouslander, JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997; 314:228.
- Bachmann G, Ebert G, Burd I. Vulvovaginal complaints. In: Lobo, RA, ed. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Philadelphia, PA: Lippincott, Williams & Wilkins, 1999:195-201.
- DiBonaventura M., Luo X., Moffatt M., Bushmakin A.G., Kumar M., Bobula J. The association between vulvovaginal atrophy symptoms and quality of life among postmenopausal women in the United States and western Europe. J. Womens Health. 2015;24:713–722. doi: 10.1089/jwh.2014.5177
- Goldstein I, Dicks B, Kim N, Hartzell R. Multidisciplinary Overview of Vaginal Atrophy and Associated Genitourinary Symptoms in Postmenopausal Women. Sexual Medicine, December 2013. 1;2: 44-53
- Vardy, MD, Lindsay, R, Scotti, RJ, et al. Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. Am J Obstet Gynecol. 2003; 189:81.
- Sabatini R, Cagiano R. Comparison profiles of cycle control, side effects, and sexual satisfaction of three hormonal contraceptives. Contraception. 2006 74:220-223
- Bachmann G, Pinkerton JA. Genitourinary syndrome of menopause (vulvovaginal atrophy): Clinical manifestations and diagnosis. UpToDate.com 2022
- Bachmann, GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician. 2000; 61:3090.
- The North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355-369.
- The North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355-369.
- Kao A, Binik YM, Amsel R, Funaro D, Leroux N, Khalifé S. Biopsychosocial predictors of postmenopausal dyspareunia: the role of steroid hormones, vulvovaginal atrophy, cognitive-emotional factors, and dyadic adjustment. J Sex Med. 2012 Aug;9(8):2066-76. doi: 10.1111/j.1743-6109.2012.02771.x. Epub 2012 May 23. PMID: 22621792.
- Nappi RE, Martini E, Cuciella L. et al. Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for Healthy Aging in Women. Front. Endocrinol. 2019. 10:561.
- Kingsberg SA, Krychman M, Graham S, Bernick B, and Mirkin S. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med 2017;14:413-424
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447
- Portman DJ, Gass ML. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014; 21:1063-1068.
- Wines N, Willsteed E. Menopause and the skin. Australas J Dermatol. 2001; 42:149-158.
- Hee, J, MacNaughton, J, Bangah, M, Burger, HG. Perimenopausal patterns of gonadotrophins, immunoreactive inhibin, oestradiol and progesterone. Maturitas. 1993; 18:9.
- Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF Jr. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008 Oct;93(10):3958-64. doi: 10.1210/jc.2008-0482. Epub 2008 Jul 22. PMID: 18647816; PMCID: PMC2579655.
- Meisels A. Menopause – A cytohormonal study. Acta Cytol. 1966; 10(1):49-55.
- Lindau ST, Dude A, Gavrilova N, Hoffmann JN, Schumm LP, McClintock MK. Prevalence and correlates of vaginal estrogenization in postmenopausal women in the United States. Menopause. 2017 May;24(5):536-545. doi: 10.1097/GME.0000000000000787. PMID: 27898651; PMCID: PMC5403603.
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998 Jan;22(1):39-47. doi: 10.1038/sj.ijo.0800541. PMID: 9481598.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014 Feb 26;311(8):806-14. doi: 10.1001/jama.2014.732. PMID: 24570244; PMCID: PMC4770258.
- Lindau ST, Dude A, Gavrilova N, Hoffmann JN, Schumm LP, McClintock MK. Prevalence and correlates of vaginal estrogenization in postmenopausal women in the United States. Menopause. 2017 May;24(5):536-545. doi: 10.1097/GME.0000000000000787. PMID: 27898651; PMCID: PMC5403603.
- Nilsson, K, Risberg, B, Heimer, G. The vaginal epithelium in the postmenopause–cytology, histology and pH as methods of assessment. Maturitas. 1995; 21:51
- Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, De Seta F, Quadrifoglio F, de Aloysio D, Guaschino S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol. 2002 Jun;40(6):2147-52. doi: 10.1128/JCM.40.6.2147-2152.2002. PMID: 12037079; PMCID: PMC130764.
- The North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355-369.
- Bachmann GA, Notelovitz M, Kelly SJ, Thompson C, Owens A. Long-term non-hormonal treatment of vaginal dryness. Clin Pract Sexuality. 1992. 8:3-8.
- Bachmann GA, Notelovitz M, Kelly SJ, Thompson C, Owens A. Long-term non-hormonal treatment of vaginal dryness. Clin Pract Sexuality. 1992. 8:3–8
- Gabrieli D, Suissa-Cohen Y, Jaber S, and Lev-Sagie A. “Modified Schirmer Test” as an Objective Measurement for Vaginal Dryness: A Prospective Cohort Study. Diagnostics. 2022, 12, 574.
- (2021) Clinical manifestations and evaluation of postmenopausal vulvovaginal atrophy. Gynecological Endocrinology; 37:8, 740-745. DOI: 10.1080/09513590.2021.1931100
- Selvaggi, SM. Atrophic vaginitis versus invasive squamous cell carcinoma on ThinPrep cytology: can the background be reliably distinguished? Diagn Cytopathol. 2002; 27:362.
- Sanchez-Borrego R. Menopausal Hormonal Therapy. In: Perez-Lopez FR, editor. Postmenopausal diseases and disorders. p. 327-348. Spinger: Chen, Switzerland, 2019.
- Smith R, Studd J. Recent advances in hormone replacement therapy. Br J Hosp Med. 1993;49:799-808.
- Reed SD, Newton KM, LaCroiz AZ, et al. Vaginal, endometrial, and reproductive hormone findings: randomized placebo-controlled trial of black cohosh, multibotanical herbs, and dietary soy for vasomotor symptoms: the Herbal Alternatives for Menopause (HALT) Study. Menopause 2008; 15:51-58
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016; 19(2):151-161.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011; 8(1):202-212
- Potter N and Panay N. Vaginal lubricants and moisturizers: a review into use, efficacy, and safety. Climacteric 2021. 24:1, 19-24
- Lev-Sagie, A, Kellogg-Spadt, S. Workshop on emollients, lubricants, enhancers, and topical hormones; making clinical recommendations. XXI World Congress In1ernational society for the Study of Vulvovaginal Disease, Paris, France, September 3-8, 2011.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis 2008; 35:512-16
- WHO Technical Report Series, No. 1025, 2020
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI360 advisory note 2012 [7 July 2015]. http://apps.who.int/iris/bitstream/10665/76580/1/WHO_RHR_12.33_eng.pdf
- Anderson L, Lewis SE, McClure N. The effects of coital lubricants on sperm motility in vitro. Hum Reprod. 1998 Dec;13(12):3351-3356.
- Markram J, Griessel L, Girdler-Brown B, Outhoff K. Sperm-friendly lubricant: Fact or fiction. Int J Gynaecol Obstet. 2022 Oct;159(1):111-115. doi: 10.1002/ijgo.14136. Epub 2022 Mar 23. PMID: 35318650
- Markram J, Griessel L, Girdler-Brown B, Outhoff K. Sperm-friendly lubricant: Fact or fiction. Int J Gynaecol Obstet. 2022 Oct;159(1):111-115. doi: 10.1002/ijgo.14136. Epub 2022 Mar 23. PMID: 35318650
- Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP. Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril. 2008 Feb;89(2):375-9. Epub 2007 May 16.
- Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP. Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril. 2008 Feb;89(2):375-9. Epub 2007 May 16.
- Lev-Sagie, A, Kellogg-Spadt, S. Workshop on emollients, lubricants, enhancers, and topical hormones; making clinical recommendations. XXI World Congress International society for the Study of Vulvovaginal Disease, Paris, France, September 3-8, 2011.
- Kabara JJ, Orth DS. Preservative free and self-preserving cosmetics and drugs. Principles and Practice. New York: Marcel Dekker, Inc; 47. 1997
- Berger KP, Kogut KR, Bradman A, et al. Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. J Expo Sci Environ Epidemiol 2019; 29:21-32
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hyroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol 2004; 24:1-4
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hyroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol 2004; 24:1-4
- Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect 2010; 86:297-302
- Willhite LA, O’Connell MB. Urogenital atrophy: prevention and treatment. Pharmacotherapy. 2001 Apr;21(4):464-480.
- Potter N and Panay N. Vaginal lubricants and moisturizers: a review into use, efficacy, and safety. Climacteric 2021. 24:1, 19-24.
- van der Laak JA, de Bie LM, de Leeuw H, de Wilde PC, Hanselaar AG. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerised cytometry. J Clin Pathol. 2002 Jun;55(6):446-451.
- Pacific Roots: https://pacificroots.com
- Chen J, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness. J Sex Med. 2013. 10:1575-1584.
- Garcia de Arriba S, Grüntkemeier L, Häuser M, May TW, Masur C, Stute P. Vaginal hormone-free moisturising cream is not inferior to an estriol cream for treating symptoms of vulvovaginal atrophy: Prospective, randomised study. PLoS One. 2022 May 12;17(5):e0266633. doi: 10.1371/journal.pone.0266633. PMID: 35551533; PMCID: PMC9098008.
- Smith R, Studd J. Recent advances in hormone replacement therapy. Br J Hosp Med. 1993;49:799-808.
- Ballagh SA. Vaginal hormone therapy for urogenital and menopausal symptoms. Semin Reprod Med. 2005;23:126-140.
- Suckling J, Lethaby A. Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2003/2006(4):CD001500.
- Suckling J, Lethaby A. Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2003/2006(4):CD001500.
- Ballagh SA. Vaginal hormone therapy for urogenital and menopausal symptoms. Semin Reprod Med. 2005 23(2):126-40.
- Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002;9: 179-187.
- Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002;9: 179-187.
- The North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355-369
- Fischer G, Bradford J. Vulvovaginal candidiasis in postmenopausal women: the role of hormone replacement therapy. J Low Genit Tract Dis. 2011; 15(4):263-267.
- Bumphenkiatikul T, Panyakhamlerd K, Chatsuwan T, Ariyasriwatana C, Suwan A, Taweepolcharoen C, Taechakraichana N. Effects of vaginal administration of conjugated estrogens tablet on sexual function in postmenopausal women with sexual dysfunction: a double-blind, randomized, placebo-controlled trial. BMC Womens Health. 2020 Aug 12;20(1):173. doi: 10.1186/s12905-020-01031-4. PMID: 32787848; PMCID: PMC7424993.
- NAMS Position Statement: The 2020 genitourinary syndrome of menopause position statement of the North American Menopause Society. Menopause: The Journal of the North American Menopause Society. Vol 27, No.9, 976-992.
- Faustino R, Perez-Lopez, NP, Vieria-Baptista P, Cohen-Sacher B, Fialho SCAV, and Stockdale CK. Management of postmenopausal vulvovaginal atrophy: recommendations of the International Society for the Study of Vulvovaginal Disease. 2021. Gynecological Endocrinology: 37:8, 746-752.
- Rioux J, Devlin M, Gelfand M, Steinberg W, Hepburn D. 17-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000; 7:156-161.
- Baker, VL. Alternatives to oral estrogen replacement: Transdermal patches, percutaneous gels, vaginal creams and rings, implants, and other methods of delivery. Obstet Gynecol Clin North Am. 1994; 21:271.
- Mandel, FP, Geola, FL, Meldrum, DR, et al. Biological effects of various doses of vaginally administered conjugated equine estrogens in postmenopausal women. J Clin Endocrinol Metab. 1983; 57:133.
- Caruso S, Cianci S, Amore FF, Ventura B, et al. Quality of life and sexual function of naturally postmenopausal women on an ultralow concentration estriol vaginal gel. Menopause. 2016;23(1):47-54
- Bachmann G, Lobo RA, Gut R, et al. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol. 2008; 111:67.
- Bachmann G, Lobo RA, Gut R, et al. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol. 2008; 111:67.
- Portman DJ, Gass ML. Vulvovaginal atrophy terminology consensus conference panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063-1068.
- Constantine GD, Simon JA, Pickar JH, et al. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause. 2017;24(4):409-416.
- Imvexxy package insert. Boca Raton, FL: Therapeutics, Inc;; 2021
- Data on file. Clinical Study Report, Protocol No. TXV14-01
- Position Statement: Bioidentical Hormones. Endocrine Society. 2006.
- Henriksson L, Stjernquist M, Boquist L, et al. A comparative multicenter study of the efects of continuous low-dose estradiol released from a new vaginal ring versus estriol vaginal pessaries in posmenopausal women with symptoms and signs of urogenital atrophy. Am J Obstet Gynecol. 1994;171:624.
- The NAMS 2017 Hormone Therapy Position Statement Advisor Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24:728-753.
- Ayton RA, Darling GM, Murkies AL, et al. A comparative study of safety and efficacy of continuous low dose oestradiol released from a vaginal ring compared with conjugated equine oestrogen vaginal cream in the treatment of postmenopausal urogenital atrophy. Br J Obstet Gynaecol 1996;103:351-358.
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause: The Journal of The North American Menopause Society. 20;27(9):976-992
- Schmidt G, Andersson SB, Nordle O, Johansson CJ, Gunnarsson PO. Release of 17-beta-oestradiol from a vaginal ring in postmenopausal women: pharmacokinetic evaluation. Gynecol Obstet Invest 1994;38:253-260.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause 2020;27:339-360.
- Naessen, T, Rodriguez-Macias, K. Endometrial thickness and uterine diameter not affected by ultralow doses of 17beta-estradiol in elderly women. Am J Obstet Gynecol. 2002; 186:944.
- Weisberg, E, Ayton, R, Darling, G, et al. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric. 2005; 8:83.
- Lee, JS, Ettinger, B, Stanczyk, FZ, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006; 91:3791.
- Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002;9: 179-187.
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol level. Climacteric 2015; 18(2):121-34.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2016. CD001500
- Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002;9: 179-187.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2016. CD001500
- Johnston S:, Farrelll SA, Bouchard D, et al; SOGC Joint Committee -Clinical Practice Gynaecology and Urogynaecology. The detection and management of vaginal atrophy. J Obstet Gynaecol Can 2004;26:503-515.
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians. Genitourinary tract changes. Obstet Gynecol 2004;104:565-615
- NAMS Position Statement: The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause: The Journal of The North American Menopause Society Vol 27, No 9, 976-992
- Bachmann G, Pinkerton JAV.Genitourinary syndrome of menopause (vulvovaginal atrophy): Treatment. www.UpToDate.com, Waltham, MA. August, 2022.
- Johnson S. Urogenital concerns. J Obstet Gynecol Can. 2006; 28:s33-42.
- The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020 Sep;27(9):976-992. doi: 10.1097/GME.0000000000001609. PMID: 32852449.
- Archer D. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-201.
- Gracia C. ACOG Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol 2014;123:202
- Faubion SS, Kingsberg SA, Shifren JL, et al. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 2020;27:976-992
- Powell BL. Identification of a 173-estradiol-binding protein in Candida albicans and Candida glabrata. Exp Mycology. 1984;8:30.
- Bachmann G, Pinkerton J.AV Genitourinary syndrome of menopause (vulvovaginal atophy): Treatment. www.UpToDate.com, August, 2022
- Cui Y, Zong H, Yan H, et al. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sexual Med. 2014;11(2):487-497
- Constantine GD Graham S, Lapane K, et al. Endometrial safety of low-dose vaginal estrogens in menopausal women: a systematic evidence review. Menopause. 2019;26(7):800-807
- Simon JA, Lin VH, Radovich C. Ospemifene Study Group, et al. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418-427
- DiDonato V, Schiavi MC, Iocobelli V, et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part II: evaluation of tolerability and safety. Maturitas. 2019;121:93-100.
- Cai B, Simon J, Villa P, Biglia N, Panay N, Djumaeva S, Particco M, Kanakamedala H, Altomare C. No increase in incidence or risk of recurrence of breast cancer in ospemifene-treated patients with vulvovaginal atrophy (VVA). Maturitas. 2020 Dec;142:38-44. doi: 10.1016/j.maturitas.2020.06.021. Epub 2020 Jul 10. PMID: 33158486.
- deVilliers TJ, Altomare C, Particco M, Gambacciani M. Effects of ospemifene on bone in postmenopausal women. 2019. Climacteric;22:5, 442-447
- Faustino R, Perez-Lopez NP, Vieira-Baptista, P, Cohen-Sacher B, et al. Management of postmenopausal vulvoaginal atrophy: recommendations of the International Society for the Study of Vulvovaginal Disease, Gynecological Endocrinology, 2021. 37:8, 746-752.
- Archer DF, Labrie F, Bouchard C, et al. Treatment of pain at sexual activity (dyspareunia) with intravaginal dehydroepiandrosterone (prasterone). Menopause. 2015;22(9):950-963
- Labrie F, Archer DF, Koitun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and the genitourinary syndrome of menopause. Menopause. 2018;25(11):1339-1353
- Labrie F, Martel C, Pelletier G. Is vulvovaginal atrophy due to a lack of both estrogens and androgens? Menopause. 2017;24(4):452-461
- Rubin R, Moyneur E, Tjoa ML, Sadiq A, Labrie F, Dury A. Prevalence of urinary tract infections in women with genitourinary syndrome of menopause and the impact of vaginal prasterone on urinary tract infections. J Urol 2020;203:e433-e434
- Holton M, Thorne C, Goldstein AT. An overview of dehydroepiandrosterone (EM-760) as a treatment option for genitourinary syndrome of menopause. Expert Opin Pharmacother 2020;21:409-415
- Fernandes T, Pedro AO, Baccaro LF, et al. Hormonal, metabolic, and endometrial safety of testosterone vaginal cream versus estrogens for the treatment of vulvovaginal atrophy in postmenopausal women: a randomized, placebo-controlled study. Menopause. 2018;25(6):641-647
- Pitsouni E, Grigoriadis T, Douskos A, et al. Efficacy of vaginal therapies alternative to vaginal estrogens on sexual function and orgasm of menopausal women: a systematic review and meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2018;229:45-56
- Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitoruinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause. 2018;25(7):837-847.
- Faubion SS, Sood R, Kapoor E. Genitourinary syndrome of menopause: managemete strategies for the clinician. Mayo Clin Proc 1017;92:18421849
- Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol 2015;33:3394-3400
- Mercier J, Morin M, Tang A, et al. Pelvic floor muscle training: mechanisms of action for the improvement of genitourinary syndrome of menopause. Climacteric. 2020;23(5):468-473
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal “rejuvenation” or vaginal cosmetic procedures: FDA Safety Communication, July 30, 2018
- Pierallli A, Bianchi C, Longinotti M, et al. Long-term reliability of fractioned CO2 laser as a treatment for vulvovaginal atrophy(VVA) symptoms. Arch Gynecol Obstet. 2017;296(5):973-978
- Gordon C, Gonzales S, Drychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause 2019;26:423-427
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and the US Food and Drug Administration Clearance: position statement. May 2016. Reaffirmed July 2018.
- Alshiek J, Garcia B, Minassian V, Iglesia CB, Clark A, Sokol ER, Murphy M, Malik SA, Tran A, Shobeiri SA. Vaginal Energy-Based Devices. Female Pelvic Med Reconstr Surg. 2020 May;26(5):287-298. doi: 10.1097/SPV.0000000000000872. PMID: 32324684.
- Shobeiri SA, Kerkhof M, Minassian VA, Bazi T; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol urodyn 2019; 38:1009-1023
- ACOG Committee on Clinical Consensus–Gynecology treatment of urogenital symptoms in indivuduals with a history of estrogen-dependent breast cancer. 2021;12:2
- Filippini M, Porcari I, Ruffolo AF, et al. CO2-laser therapy and genitourinary syndrome of menopause: a systematic review and meta-analysis. J Sex Med 2022;19:452-470
- Bachmann G and Pinkerton JAV. Genitourinary syndrome of menopause: Treatment. www.UpToDate.com, August, 2022.
- Moreno AC, Sikka SK, Thacker HL. Genitourinary syndrome of menopause in breast cancer survivors: treatments are available. Cleve Clin J Med 2018; 85:760-766. Errratum in: Cleve Clin J Med 2018;85:860
- Phua C, Baber R. The management of menopausal symptoms in women following breast cancer: an overview. Drugs Aging 2018;35:699-705
- Streicher L, Simon JA. Sexual function post-breast cancer. Cancer Treat Res 2018;173:167-189
- Goetsch M, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled clinical trial. J Clin Oncol 2015;33:3394-3400.
- Santen RJ, Mirkin S, Bernick B, Constantine GD. Systemic estradiol levels with low-dose vaginal estrogens. Menopause 2020;27:361-370.
- Constantine GD,Simon JA, Pickar JH, et al. Estradiol vaginal inserts (4 ug and 10 ug) for treating moderate to severe vulvar and vaginal atrophy: a review of phase 3 safety, efficacy, and pharmacokinetic data. Curr Med Res Opin 2018; 34:2131-2136
- Labrie F, Archer DF, Koltun W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2016;23:243-256
- Pavlovic RT, Jankovic SM, Milovanovic JR, et al. The safety of local hormonal treatment for vulvovaginal atrophy in women with estrogen receptor-positive breast cancer who are on adjuvant aromatase inhibitor therapy: meta-analysis. Clin Breast Cancer. 2019;19(6):e731-e740
- Perez-Lopez F, Phillips N, Vieira-Baptista P, Cohen-Sacher B, Fialho, S, Stockdale C. Management of postmenopausal vulvovaginal atrophy: recommendations of the International Society for the Study of Vulvovaginal Disease. Gyne Endo 2021;37(8):746-752
- Intrarosa [package insert]. Waltham, MA: Amag Pharmaceuticals; 2018
- Ke Y, Labrie F, Gonthier R, Simard JN, Bergeron D, Martel C, Vaillancourt M, Montesino M, Lavoie L, Archer DF, Balser J, Moyneur E; other participating Members of the Prasterone Clinical Research Group. Serum levels of sex steroids and metabolites following 12 weeks of intravaginal 0.50% DHEA administration. J Steroid Biochem Mol Biol. 2015 Nov;154:186-96. doi: 10.1016/j.jsbmb.2015.08.016. Epub 2015 Aug 17. PMID: 26291918.
- Goetsch MF, Lim JY, Caughey. Locating pain in breast cancer survivors experiencingdyspareunia: a randomized controlled trial. Obstet Gynecol 2014 Jun;123(6):1231-6.
- American College of Obstetricians and Gynecologists Committee Opinion. The use of vaginal estrogen in women with a history of estrogen dependent breast cancer. Number 659, March 2016.
- Ferreira E, Brown T. Therapeutics: prescription drugs. In: Belisle S, Blake J, eds. Canadian Consensus Conference on Menopause, 2006 Update, J Obstet Gynecol Canada. 2006; 28 (171):s261-267.
- https://www.fda.gov/drugs/human-drug-compounding/national-academies-science-engineering-and-medicine-nasem-study-clinical-utility-treating-patients
- Paavonen J, Brunham R. Bacterial Vaginosis and Desquamative Inflammatory Vaginitis. N Engl J Med. 2018; 379:224-54.
- Donders GGG, Bellen G, Grinceviciene S, Ruban K, Vieira-Baptista P. Aerobic vaginitis: no longer a stranger. Res Microbiol 2017;168:845-58.
- Gray LA, Barnes ML. Vaginitis in women, diagnosis and treatment. Am J Obstet Gynecol. 1965; 92:125-136.
- Gardner HL. Desquamative Inflammatory Vaginitis: a newly defined entity. Am J Obstet Gynecol. 1968; 102(8):1102-1105.
- Peacocke M, Djunkinak E, Thys-Jacobs S. Treatment of Desquamative Inflammatory Vaginitis with Vitamin D: A Care Report. Cutis. 2008; 81:75-78.
- Donders GGG, Bellen G, Grinceviciene S, Ruban K, Vieira-Baptista P. Aerobic vagi- nitis: no longer a stranger. Res Microbiol 2017;168:845-58.
- Paavonen J, Brunham R. Bacterial Vaginosis and Desquamative Inflammatory Vaginitis. N Engl J Med. 2018; 379:224-54.
- Murphy R, Edwards L. Desquamative Inflammatory Vaginitis: What is it? J Reproductive Medicine. 2008 53(2):124-128.
- Thomson, JC. Chronic Inflammation of the Vagina: Treatment and Relationship to Autoimmunity. J Reproductive Medicine. 2005; 50(7):513-523.
- Reichman O, Sobel JD. Desquamative Inflammatory Vaginitis (DIV): Twelve Year Experience with One Hundred and Thirty Patients – New Insights. Abstract presented at the International Society for the Study of Vulvovaginal Disease XX World Congress, Sept 13-17, 2009. Journal of Lower Genital Tract Disease, Supplements, October 2009.
- Peacocke M, Djurkinak E, Tsou HC, Thys-Jacobs,S. Desquamative inflammatory vaginitis as a manifestation of vitamin D deficiency associated with Crohn Disease: case reports and review of the literature. Cutis. 2010 86:39-46.
- Bradford J, Fischer, G. Clindamycin-responsive non-infectious vaginitis: a retrospective analysis of 76 cases. Abstract presented at the International Society for the Study of Vulvovaginal Disease XX World Congress, Sept 13-17, 2009. Journal of Lower Genital Tract Disease, Supplements, October 2009.
- Peacocke M, Djunkinak E, Thys-Jacobs S. Treatment of Desquamative Inflammatory Vaginitis with Vitamin D: A Care Report. Cutis. 2008; 81:75-78.
- Murphy R. Desquamative inflammatory vaginitis. Dermatol Ther 2004;17:47-9.
- Sobel JD. Desquamative inflammatory vaginitis: A new group of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol. 1994;171(5):1215-1220.
- Bradford J, Fischer, G. Clindamycin-responsive non-infectious vaginitis: a retrospective analysis of 76 cases. Abstract presented at the International Society for the Study of Vulvovaginal Disease XX World Congress, Sept 13-17, 2009. Journal of Lower Genital Tract Disease, Supplements. October 2009.
- Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. AmJ Obstet Gynecol 1994; 171(5):1215-20.
- Nirjessy P, Peyton C, Weitz M, Matthew L, and Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstetrics & Gynecology. 2006; 108( 5): 1185-1191.
- Nyirjessy P, Leigh RD, Mathew L, Lev-Sagie A, Culhane JF. Chronic “Vaginitis” in women over 50: analysis of a prospective database. Abstract presented at the International Society for the Study of Vulvovaginal Disease XX World Congress, Sept 13-17, 2009. Journal of Lower Genital Tract Disease, Supplements. October 2009.
- Newbern EC, Foxman B, Leaman D, Sobel JD. Desquamative inflammatory vaginitis: an exploratory case-control study. Annals of Epidemiology. 2002;12(5):346-352.
- Reichman O, Sobel J. Desquamative inflammatory vaginitis. Best Pract Res clan Obstet Gynaecol 2014; 28(7):1042-50
- Donders G, Ruban K, Bellen G. Selecting anti-microbial treatment of aerobic vaginitis. Curr Infect Dis Rep 2015; 17:24
- Reichman O, Sobel J. Desquamative inflammatory vaginitis. Best Pract Res clan Obstet Gynaecol 2014; 28(7):1042-50
- Reichman O, Sobel JD. Desquamative Inflammatory Vaginitis (DIV): Twelve Year Experience with One Hundred and Thirty Patients – New Insights. Abstract presented at the International Society for the Study of Vulvovaginal Disease XX World Congress, Sept 13-17, 2009. Journal of Lower Genital Tract Disease, Supplements, October 2009.
- Lev-Sagie A, Nyirjessy P. Treatment of desquamative inflammatory vaginitis (DIV) with intravaginal 2% clindamydin cream: a long term followup. Abstract presented at the International Society for the Study of Vulvovaginal Disease XX World Congress, Sept 13-17, 2009. Journal of Lower Genital Tract Disease, Supplements. October 2009.
- Wilson JF. In the clinic: vaginitis and cervicitis. Ann Intern Med. 2009; 15(5):ITC3-1-9.
- Jossens MR, Schachter J, Sweet RL. Risk factors associated with pelvic inflammatory disease of differing microbial etiologies. Obstet Gynecol. 1994; 83:989-997.
- Wilson JF. In the clinic: vaginitis and cervicitis. Ann Intern Med. 2009; 15(5):ITC3-1-9.
- Simms I, Stephenson JM. Pelvic inflammatory disease epidemiology: what do we know and what do we need to know? Sex Transm Inf. 2000; 76:80-87.
- 2007 National Hospital Discharge Survey [Internet]. Atlanta: Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/nchs/nhds.htm.
- IMS Health, Integrated Promotional Services™. IMS Health Report, 1966–2009. www.imshealth.com/
- Simms I, Stephenson JM. Pelvic inflammatory disease epidemiology: what do we know and what do we need to know? Sex Transm Inf. 2000; 76:80-87.
- Ness RB, Soper DE, Holley RL, et al. Douching and endometritis: results from PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2001;28:240-245.
- Jossens MO, Schachter J, Sweet RL.Risk factors associated with pelvic inflammatory disease of differing microbial etiologies. Obstet Gynedcol ,1994; 83(6):989-97.
- Golden N, Neuhoff S, Cohen H. Pelvic inflammatory disease in adolescents. J Pediatr.1989;114:138-143
- Kahn JG, Walker CK, Washington AE, et al. Diagnosing pelvic inflammatory disease. A comprehensive analysis and considerations for developing a new model. JAMA. 1991; 266:2594-604.
- Center for Disease control and Prevention, June, 2015 http://www.cdc.gov/std/tg2015/default.htm.
- Center for Disease control and Prevention, June, 2015 http://www.cdc.gov/std/tg2015/default.htm.
- Center for Disease control and Prevention, June, 2015 http://www.cdc.gov/std/tg2015/default.htm.
- Center for Disease control and Prevention, June, 2015 http://www.cdc.gov/std/tg2015/default.htm.
- Anastasiadis PG, Koutlaki NG, Skaphida PG, Galazios GC, Tsikouras PN, Liberis VA. Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding. Eur J Gynaecol Oncol. 2000; 21:180-3
- Lieng M, Istre P. Sandvik l. Qvigstad E. Prevalence, 1-year regression rate, and clinical significance of asymptomatic endometrial polyps: cross-sectional study. J Minim Invasive Gynecol. 2009; 16:465-71.
- Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol. 2010; 89:992092,
- Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol. 2010; 89:992092,
- Levanon, K, Crum, C, Drapkin, R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008; 26:5284.
- Levanon, K, Crum, C, Drapkin, R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008; 26:5284..
- Brown JV, 3rd, Epstein, HD, Mattison, JN, et al. Occult fallopian tube cancer in a patient with BRCA1 breast cancer. J Minim Invasive Gynecol. 2008; 15:749.
- Stewart, SL, Wike, JM, Foster, SL, Michaud, F. The incidence of primary fallopian tube cancer in the United States. Gynecol Oncol. 2007; 107:392.
- Adams Hillard PJ. The Five Minute Obsetrics and Gynecology Consult. Philadelphia, Wolters Kluwer/Lippincott, Williams & Wilkins, 2008: 100.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin .2009; 59:225.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin .2009; 59:225.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005; 106:413.
- The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. ACOG Committee Opinion No. 440. American College of Obstetricians and Gynecologists. Obstet Gynecol 2009; 114:409.
- Clark TJ, Mann CH, Shah N, et al. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial cancer: a systematic quantitative review. BJOG. 2002; 109:313.