Annotation K: Vulvar pain and vulvodynia
Click here for Key Points to AnnotationFor more on vulvodynia, go to the National Vulvodynia Association:www.nva.org.
To hear the voices of women with vulvovaginal pain, go to www.tightlipped.org.
Be sure to use the back arrow to return to this website.
This material is also found in Vulvar Pain and Vulvodynia with additional content in two other pain-related sections: Vaginismus and Pelvic Floor Dysfunction and Vulvovaginal Pain and Sexuality.
Since the 1980’s, important advances in research on vulvodynia have fostered better understanding of the intricacies of vulvovaginal anatomy and physiology and of pain itself. There has been a gradual shift away from the hope of a single cause and a single treatment for vulvodynia and an increased understanding that the condition is multifaceted, requiring an approach that considers the whole body, in all of its complexity.
In the early days of patient evaluation, we were armed only with visual inspection, a Q-tip, and the patient’s reported pain scale, with very limited knowledge about the vulva, the vestibule, the vagina, and the neurology of pain perception. Today, more health care providers are aware of the global impact of vulvovaginal pain on women’s lives, and researchers are making heroic efforts not only to understand possible causes, but also to evaluate the treatments that have been used up till now and to seek out new treatments that are based on solid evidence. Many topical and oral treatment methods previously thought to be effective for vulvodynia (at least to some extent), are now shown not to have scientific support for efficacy. Currently, studies are being assessed more stringently for validity; randomized controlled trials are encouraged, and consensus on core outcome domains for vulvodynia, that will improve the ability to perform meta analyses, is being sought.1 2
It is no small accomplishment that after forty years of sometimes conflicting research, there is strong evidence that the vulva and vagina have unique inflammatory and immunological properties. Also, specialized immunological blockers (lipids produced endogenously within the body), hold promise as a vestibulodynia treatment by resolving inflammation without impairing the host defense system.3 In August 2023 Harlow et al released a study strongly supporting the auto-immune hypothesis. They identified all women born in Sweden between 1973 and 1996 diagnosed with localized provoked vulvodynia (LPV) or vaginismus, matching each case to two women born in the same year with no vulvar pain and no ICD vulvar pain codes. As a proxy for immune dysfunction, they used Swedish Registry data to amass single organ or multi organ immunodeficiences, allergy and atopy, and malignancies involving immune cells. Women with vulvodynia, vaginismus, or both were more likely to experience immune deficiencies, single organ or multi -organ immune conditions and allergy/atopy conditions compared to controls. With increasing numbers of unique immune related conditions, risk was greater. The research suggests that women with vulvodynia may have a more compromised immune system either at birth or at points across the life course than women with no vulvar pain history. These findings reinforce the hypothesis that chronic inflammation launches the hyperinnervation that causes the debilitating pain in women with vulvodynia.4
The following section, Pain Basics, provides a simple groundwork for better understanding of how pain works in general and also in the vulva and vagina. (We have isolated the sections on Pain Basics and Pathophysiology, requiring you to open them to read them, because of their complexity, understanding that you may want to proceed directly to Diagnosis and Treatment).
Introduction
It is gratifying to lessen pain for one who suffers, and equally disturbing to be unable to help. Pain management of vulvovaginal disorders often entails, for clinicians, a comprehension beyond the level provided in their education or professional experience. Indeed, even clinicians highly experienced in diagnosing and treating patients with vulvovaginal complaints are baffled by the problem of persistent pain. Fortunately, better comprehension of the neurobiology of pain has evolved. There have been advances in functional brain imaging, further development of animal models, and the switch from a biomedical model in which nociception and pain were considered practically synonymous, to a biopsychosocial model where pain is seen as a response from the brain, and nociception can play a highly variable encoding and transmission role.
Nociception
Nociceptors are a group of sensory neurons that function as the primary unit of pain messaging. Located on A-delta and C nerve endings in skin, muscle, bone and viscera, they are endowed with receptors and ion channels that promote the detection of potentially noxious stimuli. They mediate the conversion of a sensory stimulus into an electrical signal that the brain can interpret (timing, intensity, modality, location, threshold).5 This high threshold pain signal causes action only by exposure to toxic molecules and inflammatory mediators or something intensely hot, cold, or sharp. The impulse advances to the dorsal root ganglion, then traverses to the contralateral side of the spinal cord to activate neurons in the anterior horn to produce a reflex; or the signal ascends with its message to the parabrachial area in the midbrain, then on to higher cortical centers, including the hypothalamus, the amygdala, and parts of the thalamus. The sensation of pain appears when nociceptive messages are integrated into brain networks that meld thoughts, feelings, memories, and other sensations: a cascade of molecular, cellular, electrical, and neuroimmune events. Therefore, nociception (and any other pain) becomes real only with the brain’s involvement as well as conscious awareness. Treatment must be directed to both the noxious stimuli and the brain.6
An important recent advance is recognition that the experience of pain is distinct from nociception. Nociception is the neural process of encoding noxious stimuli. Nociception is a form of messaging service which describes afferent neural activity transmission of sensory information about stimuli that have the potential to cause tissue damage. Pain is actually a complex sensory state that reflects the integration of many sensory signals with elaborate brain processing.7 In the long run, pain perception is a process mediated by the cerebral cortex. From the early perception of sensory stimuli, the dynamic spinal ascent of this information results in a nociceptive message that can be acted on, ignored, or even distorted by brain circuits.8
Over time, without further tissue injury, this state of heightened sensitivity returns to the normal baseline, where high-intensity stimuli are again required to promote nociceptive pain; the phenomenon is long lasting but not permanent.9
There is also the possibility that neurons are not alone in the development of pathological pain, (although it is still hotly debated whether vulvovaginal pain is pathologic pain). Numerous immunological pathways mediated by glia (non-neuronal cells considered part of the neural communicating system), as well as immune cells, and pro-inflammatory cytokines and chemokines modify neuronal communication leading to pathological pain.10 A full discussion of neural pathways is beyond the scope of this website. However, current pain therapeutics neglect the actions of these non-neuronal contributors. Therefore, development of interventions designed to target neuroimmune communication may improve patient outcomes.11
Peripheral and central sensitization
An additional important phenomenon that further escalates the protective function of the nociceptive system is the peripheral and central sensitization that occurs after repeated or particularly intense noxious stimuli.12 This sensitization is the result of use-dependent synaptic plasticity triggered in the central nervous system (CNS) by the nociceptor input. It represents the first example of central sensitization, discovered years ago.13
Neuronal plasticity consists of peripheral sensitization in primary sensory neurons of dorsal root ganglia and trigeminal ganglia14 and central sensitization of pain-processing neurons in the spinal cord and brain.15 Nociceptors are activated or sensitized by inflammatory mediators such as bradykinin, prostaglandins, nerve growth factor, and pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin 1β, and other proinflammatory chemokines16 that directly bind and stimulate a mosaic of peripheral receptors. The threshold for activation of the pain message lowers, yielding hypersensitivity and hyperexcitability of nociceptor neurons (peripheral sensitization) through modulation of various ion channels.17
If the peripheral nerve is damaged and the noxious messages persist, the threshold for activation of the pain message lowers, and responses to subsequent inputs are amplified, yielding increased firing of the nociceptive fibers and increased sensitivity of the A fibers.18 This is peripheral sensitization. When it occurs, allodynia in the vestibule arises from hypersensitivity to stimuli that are not normally painful. Hyperalgesia (increased response to stimuli that are not normally painful) heightens the pain.19
Similar to peripheral sensitization, central sensitization results in neural signaling within the central nervous system.20 Subthreshold synaptic signals to nociceptive neurons are recruited, augmenting action potential output: a state of facilitation, potentiation, augmentation, or amplification which is the fundamental contribution of the central nervous system in the generation of pain hypersensitivity. Because central sensitization arises from alteration of neuronal properties in the central nervous system, the pain is no longer coupled, as acute nociceptive pain is, to the presence, intensity, or duration of noxious peripheral stimuli. Instead, central sensitization yields pain hypersensitivity by changing the sensory response to normal experiential inputs, (including those that usually evoke innocuous sensations), leading to allodynia.21 Intense and prolonged painful stimuli cause an exaggerated response of excitatory and inflammatory neuropeptides, and an increased number of synapses in neurons. As was just stated, this produces neuronal excitability to noxious stimuli and innocuous (light touch) stimuli. It creates pain hypersensitivity in non-inflamed tissue by changing the sensory response elicited by normal inputs even when no peripheral pathology may be present. This sensitized state involves both spinal cord mechanisms, at the level of the dorsal horn and below, descending and supraspinal mechanisms that involve the augmented response of a network of higher brain centers. Pain is not then simply a reflection of peripheral inputs or pathology but is also a dynamic reflection of central neuronal plasticity. The plasticity profoundly alters sensitivity to an extent that it is a major contributor to many clinical pain syndromes and represents a major target for therapeutic intervention.
Descending modulation: inhibition of nociception
Central sensitization involves both spinal cord mechanisms, at the level of the dorsal horn and below, and supraspinal mechanisms that involve the augmented response of a network of higher brain centers. The sensory pain encounter can also be modified by descending modulation. The same neurons promoting top down facilitation of nociception may change to inhibition of nociception in the brain centers and spinal cord. Pain experience can be augmented or lessened depending on anticipation, attention or distraction, emotional state, mood, anxiety, coping style, outlook, tendency to catastrophize, learning, and memory. 22 The parabrachial pathway of the midbrain also projects to other centers involved in the descending control of pain, including the rostral-ventral, medulla, and the periaqueductal gray area. These descending control mechanisms are responsible for a reduction in the sensation of pain and the inhibition of its spread upon the perception of pain.23 Central sensitization may also include conditions like increased central responsiveness due to dysfunction of endogenous pain control systems (descending modulation), regardless of whether there is functional change of peripheral neurons.24
“With greater understanding of peripheral and central sensitization,and the role of neural plasticity we celebrate our progress, although clearly, more is still waiting to be learned.”25
Figures K1 A-C show a schematic diagram of a physiological model for peripheral nerve sensitization.26
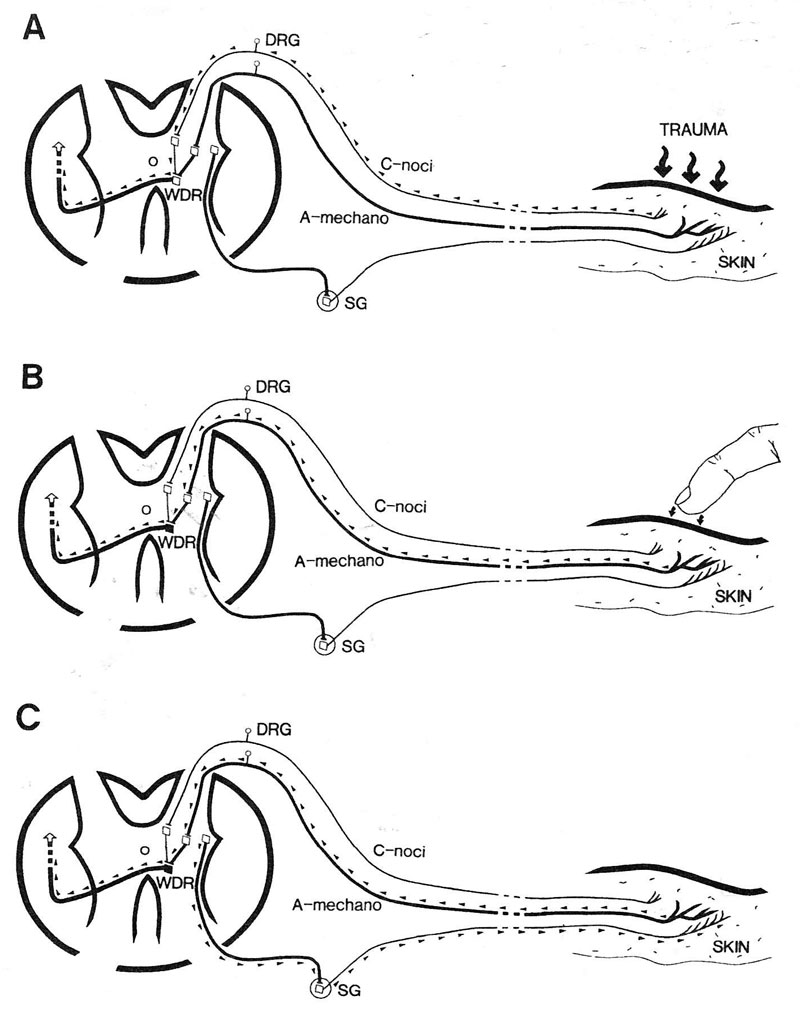
Figure K1-A shows the immediate response of nociceptive fibers to cutaneous trauma. Action potentials propagate through the dorsal root ganglion to the spinal cord to activate and sensitize wide dynamic range (WDR) neurons with axons ascending to higher centers.
Figure K1-B shows the now sensitized WDR responding to activity from touch of the large diameter A-mechanoreceptors activated by light touch.
Figure K1-C shows that with activity from central sensitization, the neurons can discharge spontaneously when there is noxious stimulus in the periphery.
Classification of pain
Without a better understanding of vulvodynia, classification of pain type is not yet clear. Over the years, several different classifications of the pain of vulvodynia have been promoted, but no one system has been accepted universally.27 Categorization, as imperfect as it is however, can still help to conceptualize different aspects of pain:
- Nociceptive Pain results from the activation of a subset of sensory neurons termed nociceptors, previously thought of as a “detect and protect mechanism,” now considered more dynamic. An important recent advance is recognition that the experience of pain is distinct from nociception. Nociception is the neural process of encoding noxious stimuli, a form of messaging which describes afferent neural activity transmission of sensory information about stimuli that have the potential to cause tissue damage. Pain is actually a complex sensory state that reflects the integration of many sensory signals with elaborate brain processing.28
- Inflammatory pain (IP) occurs after unavoidable tissue damage, e.g with joint inflammation, surgical wounds, or severe, extensive injury; sensory sensitivity is heightened to assist healing by discouraging movement or physical contact; the “dolor” reduces risk of further damage. This pain occurs as the immune system is activated by the initial tissue damage. It is low threshold pain, considered adaptive, but it mandates treatment of the ongoing inflammation.29
- Pathological pain: a) Neuropathic pain (NP) appears after development of a neural lesion or disease of the somatosensory nervous system. It is not protective but maladaptive, representing a disease state of the nervous system. This is not a warning to prevent physical injury or disease. It IS the disease—the result of neural mechanisms gone awry.30 Vulvodynia has been inaccurately called neuropathic pain, that is, disease of the somatosensory nervous system, but no neural lesion or disease of the somatosensory nervous has been demonstrated. Whether nerve injury or infection are included in somatosensory lesions needs clarification. b) Dysfunctional pain, that is, nociplastic pain,31 encompassing functional somatic syndromes (FSS), includes, irritable bowel syndrome, fibromyalgia, painful bladder syndrome, tension headaches, temporomandibular joint disease, and other syndromes of substantial pain, all without known noxious stimulus, and minimal or no peripheral inflammatory pathology.32 Since FSS include the entire group of comorbidities associated with vulvodynia, the pain of vulvodynia has also been included in the FSS or dysfunctional pain category by many experts.33 34 35 36 The concept of vulvodynia and FSS as dysfunctional pain continues to be debated.
Multiple professional societies try to contribute clarity to the process of diagnosis by creating terminology and classification systems, but this process can culminate in confusion as well as diagnosis codes. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5), published in 2013, is the primary authority for psychiatric diagnoses.37 The new classification “genito-pelvic pain/penetration disorder” in the DSM-538 is located in this compendium of psychiatric illnesses under the category of sexual dysfunctions and includes the previous terms “dyspareunia” and “vaginismus.” Vulvodynia is ostensibly there as a subset of dyspareunia, though not specifically so. Viera-Baptista, et al and others39 40 warn that there is room for misinterpretation and misunderstanding in this DSM-5 designation, saying, vulvodynia “…is not to be considered a sexual dysfunction, but rather a chronic dysfunctional pain disorder. Dysfunctional pain is defined as a disease state of the nervous system, which arises by its abnormal function, in the absence of neural structural damage.” They also point out that, “vaginismus” and “dyspareunia,” located together, can lead to confusion about the conditions and their therapeutic approaches.41 Bergeron, et al consider that this new categorization actually broadens the scope of definition for vulvodynia, as it allows for vulvar pain, deep pain, and pelvic pain on penetration.42 They mention other pain classification systems that include vulvodynia. The International Association for the Study of Pain (IASP) lists vulvodynia under “chronic primary visceral pain” within subcategories of pelvic pain.43 The 11th revision of the International Statistical Classification of Diseases and Related Health Problems has included vulvodynia within the chronic pain syndromes for the first time.44
In this website, we have addressed pelvic floor dysfunction/vaginismus separately (https://vulvovaginaldisorders.org/pelvic-floor-dysfunction/) because although there are overlaps pertaining to pelvic floor muscle tone and the psychological effects of pain or fear of pain in both vulvodynia and vaginismus, the focus of therapeutic modalities may be different.
2015 ISSVD Consensus on Terminology and Classification of Persistent Vulvar Pain
The accepted, most useful, nomenclature in categorizing vulvar pain/vulvodynia at this time is found in the 2015 consensus statement of a consortium of professional organizations on terminology that clarifies what is understood about vulvar pain from identifiable (“known”) causes and pain with unknown cause but with potential associated factors: vulvodynia.45 Click here to see the original terminology document
The addition of “potential associated factors” to the terminology initiated the deeper study of vulvodynia as a multifactorial process. We have adapted the definitions or rearranged tables below for the purpose of this document, but have stayed true to the intent of the original consensus statement.
In the 2015 consensus statement, three pain categories were identified, along with “descriptors” related to characteristics of the pain.
- Vulvar pain related to a recognizable disorder (such as infection, dermatosis, estrogen deficiency, or other identifiable condition) (see Table K1 below),
OR
2. Vulvodynia: vulvar pain of at least 3 months duration in the absence of clinically identifiable disease, (with possible associated biomedical and psychosocial factors),
OR
3. Pain with both a specific disorder (e.g. lichen sclerosus) and vulvodynia.
Although originally allocated to the diagnosis of vulvodynia alone, the following descriptors apply to both known conditions causing vulvar pain (especially those causing dyspareunia) AND to vulvodynia. 46 47
- Localized (e.g. vestibulodynia, clitorodynia, or pain in another limited location) or generalized or mixed (localized and generalized)
- Provoked (e.g. insertional, or with contact) or spontaneous (without touch), or mixed (provoked and spontaneous)
- Primary: with first tampon use or attempt at sexual penetration or secondary: developing after a period of comfortable penetration48 49
- Temporally intermittent, persistent, constant, waxing and waning, immediate, delayed, etc.
Associated biomedical and psychosocial factors that may contribute to vulvodynia have been identified (see following list) and form the basis of research into causes.50 These factors may also be seen as determining recognizable subgroups that may benefit from different treatment modalities.51 52 The word “subgroups” must be interpreted within contexts, though, as different specialists (such as neurologists and physical therapists, as well as researchers looking at the physiology of vulvodynia) may use the term uniquely within their own specialty. The “factors” will be addressed under Pathophysiology below. In some cases, there is overlap of “known” and “unknown” causes and, with future study, more factors will move into the “known” column.
- Inflammation
- Immunology
- Genetics
- Hormonal influences (e.g. endogenous or exogenous)
- Musculoskeletal (e.g. pelvic muscle weakness or overactivity, myofascial, biomechanical)
- Neurologic mechanisms
- Central (spine, brain)
- Peripheral
- Neuroproliferative
- Pudendal nerve related
- Comorbidities and other pain syndromes (e.g. painful bladder syndrome, fibromyalgia, irritable bowel syndrome, temporomandibular disorder)
- Psychosocial factors (e.g. stress, mood, interpersonal, coping, role, sexual function)
- Structural defects (e.g., perineal descent)
Localized provoked vulvodynia/vestibulodynia, (pain with light touch in the vestibule, present for at least three months, without identifiable cause, abbreviated here as LPV/PVD because both “LPV” and “PVD” are used in different studies) is the focus of most researchers because it is more common than other types of vulvar pain in premenopausal women,53 is strongly associated with female sexuality, and is therefore highly impactful for patients and their partners. Women and girls of all ages can be affected but most are in younger age groups, a high proportion under 25 years.54 55 Studies of childhood vulvodynia are few; in many cases, persistent pain is found to have an underlying cause. Despite the pain, the majority of women continue to have sex.56 Dyspareunia in postmenopausal (and lactating) women is known to be related to vulvovaginal atrophy, but more and more studies remind us that pain that persists in menopausal women using vaginal estrogen may be related to vestibulodynia.57
Generalized vulvodynia (GVD), more common in peri- and postmenopausal women,58 has not received the same attention as LPV/PVD, and there are limited specific references in evidence based literature.59 60 Localized or generalized vulvodynia may be related to varying degrees of compression of different segments of the pudendal nerve. Persistent pain after vestibulectomy may represent a pudendal nerve neuroma61 and nerve entrapment is thought to be a source of persistent genital arousal syndrome (PGAD).62 Click here for Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD)
Pudendal neuralgia (PN) is an underestimated yet important source of pain to consider during a workup. Because it is difficult to diagnose pudendal nerve-related disorders with any certainty in most women’s health practices (i.e. there is nothing visible to identify them), we have included these conditions under the category of vulvodynia, (whereas ISSVD, et al. in the 2015 consensus terminology, include them under “known causes of pain”).
Vulvodynia can disappear spontaneously, but frequently recurs. Of 239 women with vulvodynia (treatment status not indicated) in a two-year prospective study, 10% had persistent pain, and 90% experienced remission. However, half of the remission cases relapsed, often quickly. A history of longstanding pain with increased severity was more likely to persist.63
Vulvodynia burdens the health care system. Women with vulvodynia seek help from multiple health care providers, including family doctors, gynecologists, dermatologists, urologists, and alternative health practitioners. These clinicians may not be familiar with the signs and symptoms of vulvodynia, resulting in multiple visits, misdiagnoses, and delays in appropriate diagnosis and treatment. The burden not only impacts the affected woman, her family, and/or her intimate partner, but also society in general. It has been estimated that the annual economic burden of vulvodynia in the US is $31-72 billion.64 This cost includes direct health care costs, indirect health care costs (e.g., transportation to hospital), and indirect societal costs (e.g., sick leave).
Findings from available epidemiologic studies, well described in Bergeron et al’s excellent article,65 indicate that vulvodynia is a common gynecological pain syndrome, prevalent in women of all ages. To date, no global epidemiological studies have been done. In a preliminary survey, (prior to the first epidemiological study in the US), researchers showed that women from the general population were willing to provide sensitive information on lower genital tract discomfort—a first step toward bringing notice to this understudied disorder. In addition, the data supported the theory that vulvar trauma in early life may influence or serve as a marker for risk of subsequent chronic vulvar disorders.66 Subsequently, the main study of >5,000 women in Boston neighborhoods found that as many as 16% of these women experienced vulvodynia. By restricting the population to women with no lifetime histories of pelvic disorders such as endometriosis or leiomyomata, the authors conservatively estimated that at least 9% of women will experience a condition likely to meet vulvodynia criteria at some point. They continued: “If our lifetime cumulative incidence estimate is anywhere near the true prevalence, approximately 14 million US women may experience this problem in their lifetimes.”67 Another US study replicated Harlow and Stewart’s work, demonstrating that 8% of women 18-40 years old gave a history of vulvar burning or pain upon contact continuing over 3 months, limiting or preventing vaginal penetration/intercourse. The study indicated that women of Hispanic origin were more likely to develop vulvar pain symptoms than non-Hispanic white women.68 Arnold and Bachmann, et al. found a lifetime prevalence of 9.9%; 45% of these women revealed an adverse effect on their sexual experiences.69 A lifetime prevalence of 13% was shown in a recent, large study from Spain,70 and a Portuguese survey indicated a lifetime prevalence of 16%.71 However, another large study of women from Nepal, evaluated at a dermatology outpatient clinic, indicated that only 1% had vulvodynia.72 It is possible that this low number reflects patient embarrassment and reluctance to report vulvar pain or dyspareunia, or provider lack of education or disregard of the condition and discomfort with management of these women. All of these factors have resulted in widespread ignorance about this devastating condition.73
Although women of all ages may experience vulvodynia, prevalence may vary with age groups. In the USA, the National Health and Social Life Survey revealed that 21% of sexually active women aged 18-29 years experienced pain during sexual intercourse during the prior 12 months. Percentages were lower in older women: 13% of women aged 30-49 years and 8% in the 50-59 years group.74 Of 1,425 sexually active Canadian women aged 13-19 years, 20% identified pain at the vaginal opening during intercourse for over six months.75 6,777 sexually active British women aged 16-74 years, participating in a probability sample survey, showed that the prevalence of pain with sexual intercourse was highest (9.5%) in those aged 16-24 years and in those aged 55-64 years (10.4%). 76 The high prevalence in postmenopausal women in this study, compared with other studies, may arise from the inclusion of only sexually active women in the British sample. In addition, whether or not treatment for postmenopausal vaginal atrophy was being used was not addressed.77
Vulvodynia is a diagnosis of exclusion. Known disorders, that is, conditions which can be identified clearly, (although often unfamiliar to many clinicians), need to be ruled out before the diagnosis of vulvodynia can be made.78 The following table demonstrates specific disorders which can be a source of recognizable vulvar pain.
Table K-2 Possible causes of vulvovaginal pain from specific disorders (and associated links to information in this website)
| Candida vaginitis (P) | Cellulitis (Atlas of vulvar disorders) |
| Desquamative inflammatory vaginitis (DIV) (P) | Systemic diseases, e.g., Crohn (Atlas of Vulvar Disorders) Sjögren (O) |
| STIs: trichomonas (P), herpes (Herpes simplex), chancroid (Atlas of vulvar disorders ) | Fistulas (N) |
| Hypo Estrogenization (genitourinary syndrome of menopause or lactational atrophy), inadequate lubrication (P) (Atlas of Vulvar Disorders) | Drug reaction (O) |
| Irritants and allergens, irritant or contact dermatitis (J)(H) (Atlas of Vulvar Disorders) | Congenital anomalies (imperforate hymen (N), vaginal septum) (N) |
| Dermatoses with erosions, fissures (e.g. lichen sclerosus, lichen planus) (H) (Atlas of Vulvar Disorders) | Squamous cell carcinoma, other cancer and treatment for cancer, (Atlas of Vulvar Disorders), Extramammary Paget Disease (Atlas of Vulvar Disorders) |
| Dermatoses with ulcers (e.g. aphthous ulcers, pemphigus, pemphigoid) (H) (Atlas of Vulvar Disorders) | Semen allergy (Atlas of Vulvar Disorders) |
| Plasma cell vulvitis (Atlas of Vulvar Disorders) | Pudendal nerve compression or neuroma (K) |
| Trauma (obstetrical, female genital cutting) (Atlas of Vulvar Disorders) | Iatrogenic (post-op, post radiation or chemotherapy) (Atlas of Vulvar Disorders) |
| Psychosexual issues leading to poor sexual arousal, vaginismus (D) (Vulvovaginal pain and sexuality)
Vulvovaginal neoplasia (Atlas of Vulvar Disorders) |
A single cause for vulvodynia has not been identified. There is general acceptance that vulvodynia etiology is multifactorial: a varying group of influences or disorders causing similar symptoms. Studies related to the experience of pain of unknown origin also remind us that body and mind can not be separated; each biopsychosocial factor influences the others. In addition, vulvodynia research over the past two decades has revealed numerous associated abnormalities in body organs and tissues and compensatory pain mechanisms and pain pathways. Evolving information on how the brain works is just beginning to reveal new ways of understanding pain perception and processing in both vulvodynia patients and healthy controls.79 80 81
Data from these ongoing studies support the shift away from the paradigm of one cause for vulvodynia. In fact, the assumption that a blanket diagnosis of vulvodynia will direct one specific treatment is erroneous.82 It has become clear that treatment must be individualized, multimodal, and include the skills of a team of specialists. More researchers are looking carefully at the “associated factors” to direct this personalized care, as understanding them may lead to identification of vulvodynia subtypes that require different treatment modalities. (see Pathophysiology below).
Etiology of pudendal nerve-related pain (PN) is not always clear, but it is believed to result from nerve injury caused by stretching (childbirth, chronic constipation, perineal descent), entrapment, or compression (trauma, fracture of the pelvis, entrapment of the nerve between the sacrotuberous and sacrospinous ligaments or within Alcock’s canal, related to pelvic surgery, tumors, infection, chemoradiation, or, most commonly, bicycle riding).83
The current “state of the science” continues to change.84 85 The following review provides detailed information on studies related to factors associated with vulvodynia.
Introduction
In the past thirty years, the multifactorial etiology of vulvodynia, involving the complex interplay between genetic, neurological, musculoskeletal, environmental, immunological, and psychosocial factors has come into view. Failure to capture the persistently elusive causes and, therefore, the management of vulvodynia, relates to lack of basic scientific knowledge about the potentials and perils of the human vulva and vagina. Identification of “factors” associated with vulvodynia, listed in the 2015 Consensus Terminology and Classification Statement, forms the basis of historical and current research represented in this section.86 87 Review of the studies, mostly done to try to comprehend localized provoked vulvodynia/vestibulodynia (LPV/PVD), reveals evolution of thought on the conditions over time. Studies on pudendal nerve compression or entrapment show relationships to both localized and generalized vulvodynia. See Pain Basics for what is known in general about pain neurology.
FACTOR: Inflammation (regionally defined in the vulva/vestibule)
Introduction
Inflammation is not a pathological process; instead, it represents a protective immune response initiated by the evolutionarily conserved innate immune system to harmful stimuli, such as pathogens, dead cells, or irritants. Inflammation is strictly regulated by the host. Insufficient inflammation can lead to persistent infection of pathogens, while excessive inflammation can cause chronic or systemic inflammatory diseases. Innate immune function depends upon recognition of pathogen-associated molecular patterns (PAMPs), derived from invading pathogens, and danger-associated molecular patterns (DAMPs), induced as a result of endogenous stress, by germline-encoded pattern-recognition receptors (PRRs). Activation of PRRs by PAMPs or DAMPs triggers downstream signaling cascades.88
Since inflammation’s defensive mechanisms can cause pain, it has been a natural place to start investigations into the etiology of vulvodynia.
A striking feature of the inflammation of vulvodynia is that, on inspection, the condition cannot be “seen.” The classical diagnostic signs of inflammation, rubor (redness), calor (increased heat), tumor (swelling), dolor (unprovoked pain), and functio laesa (loss of function) are not present on inspection of the vulva of a woman with LPV/PVD. Mild erythema, often fleeting with light touch of the Q-tip, may or may not be present on inspection in the vestibule. However, researchers using either laser Doppler perfusion imaging or cross polarized light found enhanced blood flow, (erythema) below levels of clinical detection in the vestibules of patients with LPV/PVD, representing the possibility of inflammation.89 90 Studies are now focusing on molecular markers for inflammation to understand possible etiologies and effects of LPV/PVD: proinflammatory cytokines, neurokines, eicosanoids such as prostaglandin (PG)E2, and/or chemokines, in addition to the presence of mast cells and regional neural proliferation which have long been studied.91
Foster, et al. noted the remarkable similarity of clinical findings in patients with LPV/PVD, who present with marked mechanical allodynia in close juxtaposition to pain-free skin and mucosa.92 They suggest that the normal vulva and vagina exhibit ongoing low-grade inflammation as a defense against pathogens introduced with penetrative sexual contact or migrated from nearby body sites such as the anus.93 94
In his latest research, David Foster proposes that an inflammatory trigger, such as Candida albicans, promotes a cascade effect as follows:95
- Candida albicans stimulates…
- Proinflammatory cell migration and cellular immune response amplification in the vestibule which leads to…
- Production and release of pain-inducing substances with…
- Regional hyperinnervation of C fibers and associated TRPV1 channel increase, leading to…
- Lowering of pain thresholds, then…
- Gradual development of central sensitization, causing…
- Somatization, depression, anxiety, and hypervigilance, terminating in…
- Tightened pelvic floor muscles and increased pain, and sometimes culminating in…
- Other chronic pain disorders.
Early research studies on inflammation: 1986-2019 and Research study: Pro-inflammatory Cell Migration to the Vestibule
“Vulvar vestibulitis” was embraced as an early name for LPV/PVD, because vulvar biopsies of women with LPV/PVD showed inflammatory immune cells, mainly T-lymphocytes (related to non-specific inflammation).96 Mast cells, key players in the inflammatory response as they activate release of other inflammatory mediators, then became the object of many studies.
In 1988, small numbers of mast cells were noted in biopsies from vulvodynia cases with lower numbers in controls. The vulvar tissue showed an increased lymphocytic infiltration, considered inflammatory, in patients, with reduced infiltrate in controls.97 In 1996, a significant number of mast cells were noted in surgical (vestibulectomy) cases compared with post colporrhaphy controls. These researchers suggested a possible inflammatory association between mast cell triggering in both interstitial cystitis (bladder pain syndrome) and LPV/PVD.99 Then, 12 cases and controls studied by Chadha, et al. in 1998 revealed an increased inflammatory infiltrate, mostly lymphocytes. Plasma cells, mast cells and some monocytes were also present.100 By 2004, one of the most frequent findings across pelvic pain populations was evidence of increased mast cell count and/or mast cell degranulation, as well as nerve fiber proliferation, thought to be associated with chronic inflammation.101 102 However, non-specific inflammation was not observed in studies that followed.103 In 2007, a British study104 found no evidence of inflammation, and “no more-itis” came to the front again, with research turning its focus to pain. More recently, in 2015, Tommola et al. completed cross-sectional studies of vestibular biopsies with immunotyping of T lymphocytes, B lymphocytes, macrophages, plasma cells, dendritic cells, and mast cells. Lymphocytic infiltration density was significantly greater in vulvodynia cases compared with controls. Dendritic cells, B lymphocytes, plasma cells, and lymphoid germinal centers were greater in cases. Mast cells and macrophages did not differ between cases and controls.105 A year later, Tommola, et al. completed a second cross sectional study of vestibular biopsies from cases and controls that showed an increase in inflammatory cells (lymphocytes etc.), but mast cells and macrophages did not differ between cases and controls.106
A 2019 study now suggests that macrophages may contribute to the hyperinnervation and nociceptive sensitization of vulvodynia, and warrant further study.107 Macrophages are described as pro-inflammatory or anti-inflammatory, and can have multiple roles in the induction and resolution of inflammation. Their function can be broadly fixed, then can alter rapidly in response to the microenvironment. In addition to phagocytosis of foreign pathogens and apoptotic cells, macrophages release hundreds of effector molecules and proteins including growth factors, cytokines and chemokines.108
Continuing research studies: Changes in the vulvar biochemical milieu by regional production and release of pro-inflammatory, pain-inducing substances: cytokine, neurokine, chemokine, or prostanoid signals increase pain.
Nociceptors generate electric signals to convey the quality, duration, and intensity of noxious stimulation. Variability in nociception results from specialized receptor proteins and ion channels located on nerve endings; these identify several types of information, e.g. heat, pressure, acidity, chemicals, differentiated by the molecular markers they express.109 Ongoing nociceptive signals could alter the ion channel of these peripheral pre-terminal axons, modifying the conduction of nerve fibers, further lowering the threshold in nociceptors for pain signals,110 with ongoing elevation of pro-inflammatory substances.111 At first, assays for pro-inflammatory molecules in vulvovaginal samples had varying and inconsistent results, with some studies showing an elevation in the pro-inflammatory cytokines interleukin (IL)-1b and tumor necrosis factor (TNF)-α. Others showed lower levels of TNF-α and similar levels of IL-1b among patients and controls. In 1997, Foster and Hasday reported that vulvar fibroblasts yielded high levels of IL-6, IL-8, and prostaglandin E2 (PGE2) after stimulation by irritants in both women with LPV/PVD and controls.112 Ten years later, Foster et al went on to show that vestibular fibroblasts released elevated levels of IL-6 and PGE2 compared to fibroblasts isolated from non-painful vulvar sites. Furthermore, pro-inflammatory mediator production was elevated in LPV/PVD fibroblasts compared with controls.113 Foster’s more recent research follows under Factor: Immunology.
In summary, the evolving research into the inflammatory pathogenesis of vulvodynia has produced ample histological evidence of pro-inflammatory cell migration to the vestibule, although inflammatory infiltrates in general, and mast cell infiltration specifically, have not been found to be consistently increased in all vulvodynia studies.
FACTOR: Immunology
Introduction
The vulva and vaginal canal reveal a unique immune profile that differs from other mucosal sites and elsewhere in the periphery. There is evidence to suggest that an altered immunoinflammatory response may contribute to the etiology of vulvodynia.114
Research studies: history of allergy
The first epidemiological study to report an association between allergic reactions and vulvodynia included 239 women with and 239 women without vulvodynia. From this work, Harlow et al. suggested that women with a history of urticaria, seasonal allergies, or reaction to insect stings appear to be more prone to later development of vulvodynia than women with no history of these allergic reactions. This association was largely confined to exposures and reactions that occurred before the first onset of vulvar pain, suggesting that allergenic exposures could be a marker for or a factor involved in the development of vulvodynia. Of note, they also observed that a history of other skin conditions, not as strongly related to an immune response, generally was not associated with the risk of vulvodynia.115 Study of the recognized mediator of cutaneous urticaria intensified. Even greater attention was then focused on mast cells which release heparinase with allergic inflammation, as well as histamine, tryptase, bradykinin, and nerve growth factor (NGF) which causes nerve fiber sprouting. Increased mast cells with increased nerve fiber density in cases of vulvodynia were also reported by Bornstein, et al. 116
In 2010, Bornstein’s findings were replicated by Goetsch who found significant increases in inflammatory cell infiltrate, mast cell density, and nerve fiber density.117 A year later, a study without controls also reported increased mast cells in samples from vulvodynia patients.118 Not all studies, however, supported the presence of inflammatory cells and increased mast cell density. No difference in mast cells or the extent of inflammation was noted in 24 cases and 16 controls.119
Research study: the thymus in relationship to vulvodynia
The thymus is a mediastinal organ where young hematopoietic stem cell-derived thymocytes migrate to mature into naïve nonself-reactive T-cells. T-cells mastermind powerful, antigen specific immune activities – B cell production, and macrophage remodeling of tissues. At puberty, the thymus begins to atrophy and produces fewer mature T-cells. Pursuing the question of an association of vulvodynia with an altered immunoinflammatory system in women with vulvodynia and abnormal thymic function, researchers evaluated thymic function in cases of clinically confirmed vulvodynia and healthy controls. Their findings suggest that at younger ages, women with vulvodynia have higher thymic output and a more precipitous decline of thymic function than those without vulvodynia. It also seems that a strong immune inflammatory response is present proximate to the onset of vulvar pain and may wane subsequently over time.120
Research studies: recurrent vulvovaginal Candida albicans
Candida is both a known cause (infection) of vulvar pain, and an associated factor in vulvodynia. Various studies described findings associating vulvodynia with a possible deficient immune response that resulted in recurrent vulvovaginal Candida infections (RVVC) (more than 3 documented episodes of Candida in one year) and subsequent development of LPV/PVD. An inability to clear vulvovaginal yeast infections and the resulting chronic inflammation may lead to LPV/PVD development. (Studies supporting genetically-related findings are discussed under Factor: Genetics). Early investigations confirmed the anticipated increase in incidence of vulvovaginal candidiasis in women with LPV/PVD compared with female controls.121 122 123
Harlow et al. have shown that a positive connection exists between yeast infections prior to and after the diagnosis of vulvodynia, although the relationship varies based on the accuracy of women’s recalled diagnosis of yeast infections. Uncovering the degree of accuracy will require validation studies to determine 1) the extent to which classification of self-reported yeast infections differs between women with and without vulvodynia, and 2) whether self-reported yeast infection is a valid tool for comparative (or any) study.124 Intricate work by Foster et al.125 edifies our understanding of the complex relationship between pain and immune function with the novel observation that the vulvar vestibule (doorway to the vagina) exhibits a highly localized and tissue-specific proinflammatory response in healthy women, a response further amplified in women with LPV/PVD. The mechanisms underlying LPV/PVD need more work, despite unequivocal evidence for low-grade inflammation, altered peptidergic vulvar C-fiber innervation, and genetic susceptibilities contributing to abnormal inflammatory constellations, but Candida albicans is a known cause of inflammation across species and is implicated in more than 90% of vulvovaginal yeast infections in women. High incidence of recurrent Candida in women with LPV/PVD promoted the theory that yeast-induced vulvovaginal inflammation may underlie peripheral and possibly central sensitization of primary afferent nerve endings at the vestibule. Foster, et al. also reported that pro-inflammatory mediator production of Interleukin-6 (IL-6) and prostaglandin E2(PGE2) was robustly increased in LPV/PVD fibroblasts compared with controls. When fibroblasts were challenged with live yeast species, pro-inflammatory mediator production again rose, and the response was highly predictive of clinically measured pain thresholds.126 This work is a timely and topical advance in our understanding of site-specific mucosal immunity and also the ability to rapidly detect pathogens at a critical anatomic gateway to the reproductive tract.
It is possible that the vestibule of LPV/PVD patients is inherently more sensitive to yeast; even a subclinical Candida infection might trigger a maladaptive immune response in these fibroblasts. Foster and Falsetta’s group evaluated the signaling pathways involved in the recognition of yeast by vestibular fibroblasts that are present during chronic infection. They found that vestibular fibroblasts from LPV/PVD patients express elevated levels of Dectin-1, a surface receptor that binds C. albicans. They also demonstrated that blocking the function or expression of Dectin-1 in vitro led to a significant decrease in IL-6 and PGE2 production.127
Effective antifungal treatment, represented by a negative culture for Candida post-treatment, often does not eradicate symptoms. With that in mind, an ongoing search began for immunological and inflammatory explanations for long lasting central sensitization after resolution of acute inflammation.128
This search is further discussed under Factor: Genetics below.
FACTOR: Embryology (not a Factor in the 2015 Consensus, but references to it occur now in the literature in relationship to vulvar pain).
Introduction
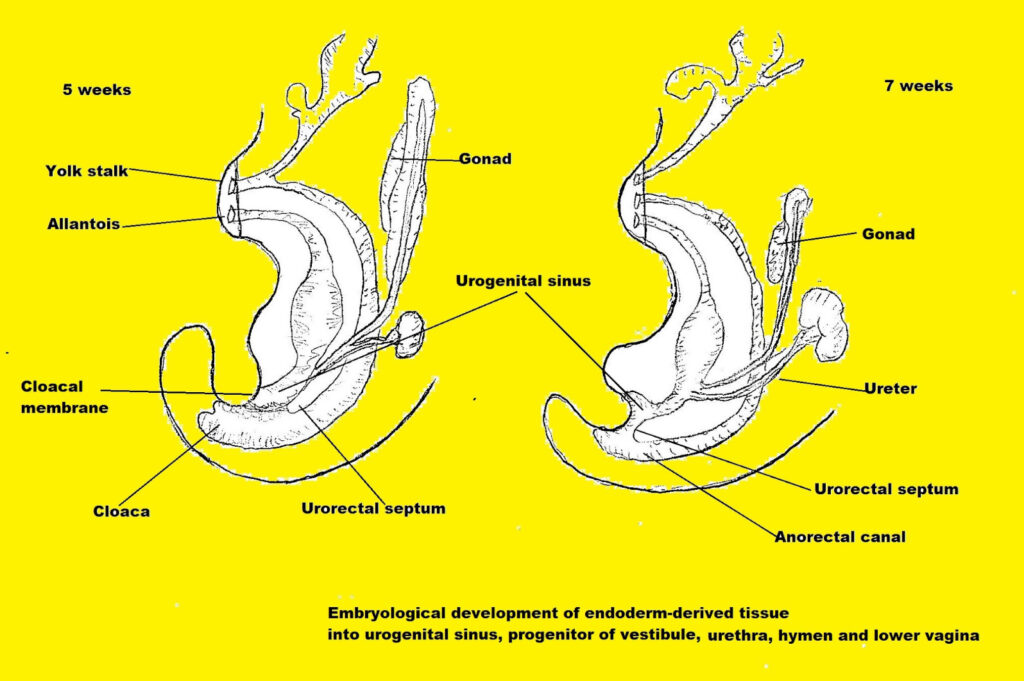
The embryological development of the vulva may be involved in the development of vulvodynia. During the fifth week of gestation, the urorectal septum divides the cloaca, creating the perineum and tissue folds on each side of the cloaca. The anterior folds form the urogenital sinus; posteriorly the folds form the anorectal canal. From the urogenital sinus comes the vaginal vestibule, where the urethra, vagina, and greater vestibular glands open. It has been hypothesized that the endoderm-derived tissue comprising the vestibule, urethra, hymen, and lower third of the vagina has special immunologically protective characteristics.129 130
More than one scientist has proposed that these tissues, all derived from the embryological urogenital sinus, are immunologically unique, a protective doorway to the vagina with special defensive powers.131 132 133 (The hymen, part of the endoderm-derived group, has no known anatomical or physiological function, although prepubertal protection from infection has been hypothesized).134
Cases of interstitial cystitis co-existing with vulvodynia have been reported, raising the question of whether the two painful conditions could represent a congenital anomaly: a disorder of urogenital sinus-derived endothelium.135 Other structures derived from endoderm, including bladder and anterior urethral wall may also exhibit unique immune properties.136
FACTOR: Genetics
Introduction
Genetics is an emerging discipline in relationship to vulvodynia; it remains to be seen whether all the answers we expect regarding chronic pain from genetic sources materialize. Genetic interactions, genetic-environment interactions, and epigenetic variants can involve multiple genes. At this point it is difficult to reliably estimate the genetic component of chronic pain conditions because of the complexity of these interactions.137
Research studies: genetic studies revealing polymorphisms
Reliably estimating the role of the emerging and complex discipline of genetics in vulvodynia is an arduous undertaking. Genetic interactions can involve multiple genes, as well as genetic polymorphisms (variations of the gene) and epigenetic variants, (heritable changes that do not affect the DNA sequence but change gene expression, e.g., addition of a methyl group to part of the gene molecule).138 To date, genetic studies related to vulvodynia have focused on several possible mechanisms that could form a foundation for genetic predisposition:139
- Genetic polymorphisms that increase the risk of candidiasis or other infections
- Genetic changes that permit prolonged or exaggerated inflammation
- Altered immunological response
The role of genetics in common chronic pain conditions suggests minor contributions from a large number of single nucleotide polymorphisms representing different functional pathways. 140
A genetic profile of women suffering from vulvodynia, especially looking at genetic polymorphisms from genes coding for cytokines, interleukin-1 receptor antagonist and interleukin-1 beta, and gene coding for mannose-binding lectin (MBL), is discussed below. These polymorphisms result in a stronger inflammatory response, making these women highly susceptible to pain.141
In 2000, Jeremias et al. noted a higher frequency of certain alleles in interleukin-1B and interleukin-1 receptor antagonist polymorphic genes among women with vulvodynia.142 Clinical and laboratory-based studies then showed that women with vulvodynia exhibit lower serological levels of interleukin-1 receptor antagonist. This finding suggests that these women may be at a greater risk of a proinflammatory immune response resulting from their inability to terminate an inflammatory event involving IL-1 production.143 The same researchers confirmed their findings with another genetic study of IL-1.144
In the innate immune system which comprises the initial defense against microbial invasion, a major component of antimicrobial innate immunity is mannose-binding lectin (MBL). MBL is present in the systemic circulation.145 146
NALP3 inflammosome and mannose binding lectin (MBL) represent an innate immune system of receptors/sensors that regulate the activation of caspase-1. This enzyme proteolytically activates the pro-inflammatory cytokines interleukin-1 beta (IL-1 beta and IL-18.). MBL and the NALP3 inflammasone induce inflammation in response to infectious microbes and molecules from host proteins.147 NALP3 inflammosome and MBL are also associated with defense against Candida species. The genes that code for each are polymorphic. Women with LPV/PVD had a higher MBL variant allele frequency than controls. MBL specifically binds to mannose and N-acetyl-glucosamine molecules on microbial surfaces, initiating complement-dependent microbial killing and microbial opsonization via MBL-recognizing receptors on the surface of macrophages and dendritic cells.148 149 Individuals with decreased circulating levels of MBL were shown to possess variant alleles in exon I of the MBL gene. A single nucleotide substitution at codon 54 was associated with an increased rate of infection.150 Subsequent investigations, however, have shown conflicting results.151
FACTOR: Hormonal influences
Introduction
Gonadal hormones are thought to be indispensable cornerstones of normal development and function, and it appears that no body region, no neuronal circuit, and virtually no cell is unaffected by them. Thus, increasing awareness toward estrogens appears to be obligatory. 152
Research studies: estrogen and nociception
Estrogen is involved in multiple roles in reproduction and gynecology not covered here. Sensation and nociception occur at the peripheral, spinal, and supraspinal levels.153 In the periphery, rapid 17-beta estradiol signaling of membrane bound ER-alpha and -beta is important in mechanical nociception.154 Nociceptor signaling may vary across the menstrual cycle as hormone levels fluctuate. High levels of circulating estrogen during the preovulatory period enhance structural integrity of tissue and estrogenic inhibition of purinoreceptors (P2X3s) may allow genital tissue to withstand the physically rigorous act of intercourse.155
A wide array of functions can be attributed to estrogens, including the dramatic change from non-painful to painful mechanical pressure. This pain threshold is encoded by voltage-gated calcium channels (VGCCs) and purinoreceptors (P2X3s) located near nociceptive nerve endings.. The rapid switching message from non-painful to painful mechanical pressure encoded by the P2X3s activates the rapid release of adenosine triphosphate (ATP), resulting in a rapid inflammatory response. Inflammation alters the voltage dependence of the P2X3 receptors and enhances their activity, boosting neuronal hypersensitivity and pain. Reduced mechanical pain thresholds may reflect inadequate ER-alpha regulation of P2X3, leading to excessive activity of P2X3 and more pain. Support for this hypothesis includes data indicating that postmenopausal women show that tissue depletion of estrogen is related to mechanical allodynia.156 157 158
Depletion of female gonadal hormones in rodents induces mechanical and thermal hypersensitivity that parallels a threefold increase of P2X3 receptor expression in the dorsal root ganglion.159
Results of diminished or blocked estrogen- what happens when there is not enough?
It is well known that vulvar and vaginal tissues are responsive and dependent on sex steroids for integrity and function; a deficiency in circulating estrogen leads to changes in the anatomy and physiology in the vagina. Both natural and iatrogenic causes of decreased sex steroids can lead to symptomatic physiologic change. Clearly, the most common cause of lowered sex steroids in women is menopause. Lactational anovulation, anorexia with hypothalamic amenorrhea, and hyperprolactinemia are other important natural causes.160 Surgical factors including oophorectomy and hysterectomy without oophorectomy come into play, as well.161 American women also have a lifetime risk of being diagnosed with breast cancer; 70-80% of breast cancer tumors express the estrogen receptor (ER) and/or the progesterone receptor. Long term systemic anti-estrogen therapy is often an indicated therapy. The regimen usually consists of a selective estrogen receptor modulator (SERM) such as tamoxifen for premenopausal women or an aromatase inhibitor (AI) for postmenopausal women. Both classes of drugs suppress circulating estrogen, possibly predisposing patients to dyspareunia.162
Spironolactone, another hormone modulating treatment for androgen mediated cutaneous disorders (AMCD) such as hair loss, hirsutism, and acne, is another possible contributor to dyspareunia. Spironolactone is an androgen antagonist which decreases the effect of endogenous androgen by inhibition of testosterone and dihydrotestosterone binding to androgen receptors. While approved for the treatment of hypertension, spironolactone is increasingly used in the treatment of AMCD. A recent case report has linked spironolactone with hormonally associated vestibulodynia and decreased sexual arousal. Another 17-spironolactone chemically similar to spironolactone is the progestin in the oral contraceptive Yasmin. Yasmin has been shown to reduce the frequency of intercourse and orgasm, and possibly introduce hormonally associated vulvodynia.163
Research studies: sex steroids and vestibulodynia: The role of CHC’s and LPV/PVD
A great deal of research has focused on the possible influence of combined hormonal contraceptive (CHC) use on increased risk of vulvodynia. A polymorphism in the androgen receptor significantly raises the risk of developing combined hormonal contraceptive-induced LPV/PVD.164 CHC formulated with an estrogen and a progesterone lead to a lower serum estradiol and free testosterone by decreasing ovarian production of estrogen and total testosterone and by inducing the liver to produce increased levels of sex hormone binding globulin (SHBG). In addition, some CHCs contain synthetic progestins that act as testosterone antagonists at the androgen receptor.165
In addition, it has been shown that CHCs induce morphologic changes in the vestibular mucosa, raising its vulnerability to mechanical strain.166 CHC use has also been associated with decreases in mechanical pain thresholds167 and with decreasing clitoral size, labial thickness, and introital diameter. All of these reductions led to decreased orgasm, lessened sexual frequency, limited lubrication, and increased dyspareunia associated with CHCs.168
It follows, with all the effects on sex steroids, that researchers have looked for a connection between use of CHCs and development of LPV/PVD. Women who used CHCs before the age of 17 years had a relative risk of 11 of developing LPV/PVD.169 Both Bouchard, et al.170 and Harlow, et al. confirmed in case-controlled studies that early CHC use significantly increases the risk of developing LPV/PVD.171 In one study, use of CHCs containing ethinyl estradiol at a dose of <20 mcg significantly increased the risk of LPV/PVD.172 There is also a case series of 50 women who developed LPV/PVD while on CHCs; treatment with topical estradiol and testosterone was successful after cessation of the CHC.173 However, there is some conflicting evidence about the role of CHC, with one population-based study of 906 women showing no increase in risk of vulvodynia.174
Research studies: estrogen and postmenopausal pain
Attention to lack of estrogen and postmenopausal dyspareunia led to the first study to systematically investigate the clinical attributes of dyspareunia pain (location, sensory quality, and intensity) in postmenopausal women, demonstrating that, similar to premenopausal dyspareunia, it is a heterogeneous condition. A study of 182 postmenopausal women 45-78 years old, found that by looking at all aspects of the pain, six subgroups could be identified. Clusters V1, V2, and V3 featured pain confined to the vulvar vestibule. Clusters V4-V6 experienced genito-pelvic pain. The predominance of provoked vestibular pain (97.8%) was consistent with epidemiological and clinical studies reporting that superficial dyspareunia is the most frequently found subtype in postmenopausal women. However, pain in other genital and pelvic locations was noted in a significant proportion (22-32% in the patient and 8.5% in the general population). Hence, the familiar division of superficial and deep dyspareunia in postmenopausal women is not supported. Rather, pain may be restricted to the vulvar vestibule, or occur in combination of vestibular and other genital and pelvic sites. And this group V1 may be derived from classic LPV/PVD in menopausal women, while the V2 group may be showing the clinical attributes associated with vulvovaginal atrophy. The study is limited by its lack of controls.175
Another study of pain in postmenopausal women emphasizes that all postmenopausal vulvar pain cannot be attributed to vulvovaginal atrophy. Using data from 371 participants ages 42–52 years at the Michigan site of the Study of Women’s Health Across the Nation (SWAN), researchers aimed to provide population-based information looking at the associations of vaginal symptoms, serum hormone levels and hormone use. 40% of the women reported chronic vulvar pain; 13.7% indicated past but not current pain, or short duration vulvar pain symptoms. Of those with chronic pain, 25% did not report dryness. Use of hormones during the preceding year was more likely in women with current, chronic, and past/short duration vulvar pain symptoms (13.3%, 17.6% and 20% respectively: p < .01). With each log unit drop in serum dehydroepiandrosterone-sulfate and testosterone levels, there were increased relative odds of current vulvar pain symptoms. Despite the possibility of inadequate hormonal treatment in some cases, vulvar pain unresponsive to hormone therapy, supports the presence of a chronic pain condition, such as vulvodynia, that mandates alternative, non-hormonal treatment measures. The most common complaint associated with vulvovaginal atrophy, vaginal dryness, was not reported by >25% of the women with current chronic vulvar pain. Elevated odds of reporting chronic vulvar pain were associated with lower average DHEA-S and testosterone levels prior to the presence of vulvar pain symptoms. These findings yield increased evidence that chronic vulvar pain in postmenopausal women has a multi-variant etiology, and may not be explained by estrogen deficiency and atrophy alone. Postmenopausal women may be experiencing new onset, exacerbated and/or long-term chronic vulvar pain consistent with vulvodynia. A trial of hormone therapy is advised for women with both pain and atrophy, with alternative vulvodynia treatments in those who have no pain improvement with hormonal therapy.176
From 2008-2016, Goetsch, et al. followed sixteen cases of burning vulvar pain that remitted with prolonged estrogen in postmenopausal women 44-85 years old. 9 women had premenopausal oophorectomy. Each patient received nightly estradiol either as a topical cream to the vestibule, or continuously by transdermal patch. They were followed by serial examinations and telephone contact follow-up over eight years. The women were taught how to apply local 4% lidocaine prior to the estradiol if needed. Continuous estradiol use was associated with reduced pain in all patients, with slow progression of improvement. Prolonged therapy over an average of eight months eliminated the incessant burning pain in 12 (70%) women. Four women reached a plateau with pain scores of 2-3 out of 10. Two women required mucosal excision after estradiol therapy had improved them initially. The author points out the marked trophic effect on genital pain nerves related to lack of estrogen; years of gradually mounting nerve growth may require much longer and stronger treatment to prune nerves and regenerate normal function. She raises the concern that avoidance of estrogen may have long-term consequences for urogenital tissue.177
Liao, et al. have elucidated the physiology of vestibular nerve sprouting in premenopausal women. A local renin angiotensin system generates a local trophic stimulus achieving angiotensin II receptor activation in mechanoreceptors, spawning nerve proliferation.178
Examination of vaginal tissues in women undergoing anterior colporrhaphy revealed that densities of both autonomic and sensory nerves in the vaginal submucosa are related to estrogen status.179 All women were free of vaginal pain; tissues showed greatest nerve densities when there was no estrogen exposure, moderate densities with systemic estrogen therapy, and fewest with topical estradiol therapy. We also need to recognize that increased autonomic innervation may influence atrophy by way of vascular supply, mass and smooth muscle tone in postmenopausal vaginas, distinct from the pain consequences of increased nociceptor densities.
The unevenness demonstrated by these women may appear to be a morass of presentations and responses, but is really a status quo for end-organ sensitivity to estrogen, a well known idiosyncrasy of the hormone. It would appear that the criteria for vestibular homeostasis varies widely among women.180
The common complaints in 50% of postmenopausal women with dyspareunia have always been attributed to vulvovaginal atrophy. The current term Genitourinary Syndrome of Menopause (GSM) covers more symptoms than atrophy alone and promotes a more detailed exploration of the dysfunctions occurring in menopausal women. Reports from postmenopausal women of symptoms affecting multiple regions of the genitourinary tract are common. While the FDA authorizes local estrogen cream therapy to be inserted in the upper vagina, one wonders how helpful intravaginal estradiol is for the labia, introitus, urethra and bladder. And often inadequate attention is paid to other diagnoses that may contribute to dyspareunia such as tenderness of pelvic floor muscles, bladder, and uterus or adnexa.
A 2022 study181 aimed to describe the symptoms and physical examination findings in a cohort of women with moderate to severe dyspareunia being screened for a clinical trial of two strengths of estradiol cream. For 55 women, a modified vulvar Pain Assessment Questionnaire (VPAQ screen) and a 13 item questionnaire created from several validated instruments were used. The Urinary Distress Inventory, the Sandvik Severity Index, the O’Leary/Sant Interstitial Cystitis Symptom Index, and the Urinary Tract Symptom Assessment were employed to obtain symptom information. Physical exam and standard pelvic exam were performed along with the tampon test and cotton tipped swab test. 4% lidocaine was applied to all the vestibule for 3 minutes; then the cotton swab test was repeated and scored by NRS. Pelvic floor muscles were evaluated.
Mean age of the women was 59.5 years. Mean pain scores with intercourse was 7.3 out of 10 by numeric rating scale. 30 of 55 women had stopped intercourse for lengthy periods due to pain. 70% of the women had used estrogen after menopause, citing dyspareunia in most cases. 70% of users reported failure to obtain relief.
49% of women reported symptoms affecting the outer vulva, by use of a detailed drawing. Dryness was the most common symptom (58%), while 20% reported fissures, splits, or tears. Lower urinary tract symptoms occurred in 45 women (82%).
Physical findings in the majority were consistent with vulvovaginal atrophy. Tenderness was localized to the vulvar vestibule. Fifty of 55 women (91%) reported pain >3 at some location adjacent to the hymen. Examination tenderness was fully extinguishable in every participant with a 3 minute application of 4% lidocaine. Mean vaginal pH was 5.6. Pelvic floor muscle tenderness was the second most common location of tenderness.
Burning was the most frequently reported descriptor. Dryness presented a dilemma in interpretation. It is not a listed descriptor in the McGill Pain Questionnaire; it is a descriptor in the VPAQ. Because the term “dry” is the most common symptom attributed to VVA/GSM participants were requested to score “dry” as a descriptor of intercourse. Along with “burning,” “dry” was a similarly frequent descriptor endorsed by this cohort of women for both spontaneous pain and insertional pain.
A minority of studies have assessed the consequences of estrogen deficiency in the nerves of the human genital tract. The epithelium of the human vulvar vestibule is richly innervated,182 with documented increases in these nerves in postmenopausal dyspareunia, confirmed to be sensory nerves in LPV/PVD.183 In contrast, the innervation of the human vagina consists of submucosal autonomic nerves and only rare pain nerves. It is this vestibular innervation with sensory nerves that yields a credible explanation for the tender vestibular tissue reaction to topical lidocaine.
This finding that the primary location of genital tract tenderness is the vulvar vestibule promotes consideration of targeted therapy to the introitus as a treatment for postmenopausal dyspareunia. Findings that topical lidocaine extinguishes vestibular tenderness indicate that the study of superficial nociceptor function in the GU tract may lead to an improved understanding of the underlying mechanisms of dyspareunia. Increased focus in GSM research on specific GU structures may help assign common complaints to locations and lead to recognition that symptoms vary and relate to the duration and degree of estrogen deficiency. More precise therapy regimens may thereby emerge.184
FACTOR: Neurologic mechanisms
Introduction
Advances in understanding vulvodynia have resulted from the study of neurology and pain. The intricacies of vulvovaginal sensation, steps toward understanding neural circuitry, peripheral and central sensitization, and the critical role of the central, peripheral, and supraspinal regions in the interpretation of pain: all have contributed hugely to advance our work.
From the beginning it is important to recognize that the nervous system is not a static system.
Neuroplasticity is a process that is making neural pathways more difficult to delineate. It involves the adaptive ability of the nervous system to change its activity in response to intrinsic or extrinsic stimuli by reorganizing its structure, functions, or connections after injuries. 185
Research studies: Painful genital sensation: considering subgroups of vulvodynia.
Studies related to LPV/PVD have focused primarily on pain limited to the vulvar vestibule, despite the understanding that vaginal muscle tone is either affected by vestibular pain or contributes to it.186 187 Some researchers, however, are looking into associated anatomical sites, hypothesizing that their contribution to the diagnosis of vulvodynia is being overlooked. Falsetta and Foster, et al, proposed that the vestibule is uniquely immunologically defensive to pathogens, causing inflammation that might lead to pain.188 189 Farmer, in 2013, also drew attention to the special immunological characteristics of the vestibule, in its position as gateway to the vagina.190
This research draws attention to the question of whether the term vestibulodynia is a limiting one. Farmer explores evaluation in her controlled trial, of pressure applied to the vagina as well as to the vestibule, finding that patients with LPV/PVD experience pain with low pressure in the vagina in comparison with controls whose pain sensation occurs under much more pressure.
Vulvar pain is both a somatic and visceral disorder. Pain hypersensitivity promoted by light mechanical vulvar touch (vulvar allodynia) is characteristic of provoked vestibulodynia (LPV/PVD). Pain brought on by distension of the vagina by digital, penile, or mechanical penetration (vaginal allodynia) destroys the pleasure of sexual intercourse, makes tampon use impossible and fosters a search for relief. Pain in the vestibule in some women can be caused by slight touch (sitting or tight-fitting clothing), whereas in others it is caused only by vaginal penetration during sexual intercourse, tampon insertion, or gynecological examination, resulting in dyspareunia or a complete inability to have intercourse.191
Dyspareunia involves more than vulvar hypersensitivity. Vaginal allodynia is seldom mentioned in LPV/PVD research, even though we have learned that pain with intercourse is not necessarily alleviated with relief of experimentally induced vulvar pain.192 It is not yet known whether vulvar mechanical allodynia is a suitable analog to natural vaginal distension, a predominant source of LPV/PVD pain.193
Measuring static mechanical allodynia requires the correlation of discrete stimulus intensity to a perceived magnitude of pain. In 2013, to study psychophysical properties of pain, Farmer et al measured static (fixed) as well as dynamic (time varying) characteristics of somatic and visceral genital sensation in women with LPV/PVD and matched controls. Women judged varying degrees of painful and non-painful sensation of the vulva and vagina. A linear potentiometer with a spring calibrated to exert the desired pressure was used for vulvar testing. Vaginal distention was achieved with physiotherapeutic dilators.194 All women were provided with experience in rating different intensities of vulvar pain and vaginal sensation so that variability in individual perceived genital sensation would be constant across participants.
Women used a hand attached device to indicate the amount of discomfort experienced with a touch or pressure stimulus to complete a systematic comparison of experimental vulvar touch vs vaginal distension pain in the effort to determine whether punctate pressure testing accurately simulates the naturalistic pain of women with LPV/PVD during intercourse in comparison to healthy women. The distinction between vulvar touch and pain is largely determined by the stimulus intensity, with substantial interindividual variability in the amount of force required to elicit pain. The control group (“normal women”) could tolerate greater amounts of force without perceiving vulvar pressure to be painful. In addition, the mean slope values that show the stimulus-response relationship for vulvar touch and pain were roughly equivalent in normal women whereas individual slopes in women with LPV/PVD showed greater pain perception, sooner, than in controls. Increases in pressure, whether painful or not, and the subjective quality of vulvar pain were appraised in a similar manner. (See next study). The same adjectives used frequently by women with LPV/PVD: “burning“ and “sharp,” were used by healthy women, raising the possibility that provoked vulvar touch, pressure, and subjective quality of pain feel quantitatively similar.195
Research studies: Sensory deviations in perception of vulvar pressure and vaginal distension in LPV/PVD
Study of vulvar pressure and vaginal distension permitted discrimination between sensory characteristics of innocuous genital somatic (vulvar) touch and innocuous visceral (vaginal) distention. Similar to vaginal distension in animal models, researchers found that healthy women could reliably differentiate between perceived levels of fullness with varying dilator volumes, suggesting transmission of innocuous stimuli. Their ability to recognize variation accurately in vaginal fullness exceeded their discrimination of vulvar touch levels. The difference in vulvar and vaginal discrimination shows that in healthy women vaginal distension perception reflects a distinct component of visceral sensation, resulting from dilator insertion. LPV/PVD patients showed a markedly slower rate of perception of non-painful touch in comparison to touch perceptions in controls. Yet both controls and LPV/PVD subjects show compatible time constants and thresholds in response to painful stimuli. LPV/PVD women identify fullness at significantly reduced distension volumes compared to controls and compared to LPV/PVD vaginal pain perception. “Similar to the stimulus-perception patterns for vulvar touch perception, women with PVD required lesser amounts of vaginal distension to perceive equivalent perceptions of vaginal fullness compared to controls.”196
Research studies: Stimulus thresholds in the onset of vulvar and vaginal perception
No group differences were found in the vulvar pressure required to elicit touch. A significantly lower distension volume was required to elicit vaginal fullness perceptions in women with LPV/PVD compared to controls.
As with animal vaginal distension data, Farmer et al. identified the ability in healthy women to accurately determine the differences between perceived levels of fullness with varying dilator volumes, suggesting transmission of innocuous stimulus information. And, remarkably, their ability to discern variation in vaginal fullness was superior to their discrimination of levels of vulvar touch.
The pain of vaginal distension arises from recruitment of primary visceral afferent circuitry and some pudendal somatic innervation near the introitus.197 Vaginal distension perception, as mentioned above, demonstrates a distinct component of visceral sensation, different from that of vulvar stimulation from insertion of a dilator. In animal studies, regional differences in sensory acuity are a familiar finding; vaginal visceral sensation has been characterized as diffusely localized and less temporally distinct than somatic. The capacity of a healthy woman to accurately discriminate levels of vaginal distension promotes the novel concept that sensory properties of the vagina differ from those of other visceral structures.198
Research studies: Clinical parameters associated with genital perception in LPV/PVD
Women with LPV/PVD perceived vulvar touch and pain in response to far less pressure than healthy women. Static sensory measures are therefore adequate to confirm LPV/PVD pain as a form of vulvar allodynia and vulvar hyperalgesia. Because this heightened pain perception is relative to the peak stimulus intensity, it is possible that this early peak reflects the cognitive and emotional responses to the experience of pain, rather than the magnitude of nerve fiber activation at the vulva.199
Research studies: Regional hyperinnervation of C fibers
Hyperinnervation of the vestibule in women with LPV/PVD, has been studied in search of the etiology and physiology of the condition.200
Hyperinnervation of nociceptive nerve fibers in the vulvar vestibule was originally identified by Westrom and Willen; however, they noted no significant correlation with the presence or absence of pain.201 Within the same year, Bohm-Starke, et al. also identified the nerve fiber increase.202 They used immunohistochemical methods to confirm Westrom’s findings of these nerve endings as nociceptors.204 The increase in density of vulvar nociceptive fibers was thereafter confirmed by others.205 206
The vanilloid receptor VR1 (TRPV1) is expressed by nociceptors, and is triggered by capsaicin, noxious heat, protons, and chemicals produced during inflammation. Tympanidis, et al. reported that an increase of vanilloid receptor VR1 innervation in vulvodynia may contribute to sensory symptoms experienced by these patients.207 In a follow up study, Tympanidis et al. showed increased papillary VR1 fibers by immunostaining and image analysis in vulvodynia tissues compared to controls. VR1 expression was found to be significantly increased. VR1-positive fine epidermal fibers also appeared to be increased in vulvodynia tissues, by inspection. Fibers immunoreactive to the voltage-gated sodium channel SNS1/PN3, also expressed by nociceptors, were relatively scarce in both vulvodynia and control tissues.208
Leclair, et al showed that women with primary vestibulodynia have significantly greater neural proliferation as compared to women with secondary vestibulodynia, supporting the hypothesis that each may have its own distinct histopathologic pathway, rather than representing different stages of the same disease.209
Researchers have also studied the relationship of inflammatory cells associated with the renin-angiotensin system (RAS) to regional hyperinnervation in LPV/PVD cases. They discovered that vestibular tissue from these women revealed increased numbers of mechanoreceptive nociceptor axons as well as T cells, B cells, and macrophages. There was no difference in mast cells between the groups. In the LPV/PVD cases, the inflammatory cellular infiltrate expressed increased levels of RAS proteins. ANG II (angiotensin II) production was regionally increased in LPV/PVD cases, ANG II protein promoted neural growth in LPV/PVD cases, and finally, inhibition of ANG II or a biologically related peptide, appeared to block this neural proliferation process.210
As researchers begin to look at neuroproliferation in the pathogenesis of LPV/PVD, the normal, non-pathological anatomy and innervation of the vestibule need thorough review, with consolidation of current evidence. Such a study would promote understanding of vulvar vestibular function, its role in development and persistence of life-changing pain syndromes, and its influence on development of improved treatments for women with LPV/PVD.
To learn more related to vestibulodynia, vestibular anatomy, and innervation in pathological vulvar vestibular tissues from women with vestibulodynia, researchers selected 15 original research articles published from 1998 to 2017.211 Microscopic patterns of innervation of the vulvar vestibule by histology and immuno-histochemical (IHC) techniques from twelve of the studies are shown in Figure K3, a schematic representation of the non-pathological (normal) and pathological innervation of the human vulvar vestibule.The illustration highlights the nerve fiber types identified in the vulvar vestibule with their associated immunohistochemical markers. General, nociceptive, parasympathetic, sympathetic, and mechanosensory innervation has been identified in non-pathological (left) and pathological (right) tissue. Current evidence indicates hyperinnervation of general and nociceptive nerve fibers in pathological tissues. Immunohistochemical markers that display hyperinnervation in pathological tissues are marked with an asterisk. This illustration is a summary of current findings, and does not represent the anatomical location, distribution, or density of the innervation.
FIGURE K3 (used with permission of John Wiley & Sons)212
To summarize, increased density of nociceptive nerve fibers was noted early in the study of LPV/PVD. Researchers have identified all the known nerve fibers in LPV/PVD. VR1-positive fine epidermal fibers are increased in LPV/PVD. Greater nerve proliferation occurs in primary LPV/VPD versus secondary. Inflammatory cells associated with RAS express increased levels of RAS proteins. ANG II is increased in LPV/PVD cases and promotes neural growth. Hopefully, inhibiting ANG II may block this neuroproliferative process.
FACTORS: Neurologic mechanisms and comorbidities
Research studies: Regional lowering of thresholds to varied stimuli leading to comorbidities with other pain syndromes
Remarkably uniform marked mechanical allodynia (pain produced by a stimulus that does not usually induce pain) of the vulva, located millimeters away from pain free skin and mucosa of the vulva is the defining characteristic of localized provoked vulvodynia.213 Allodynia of the vulvar vestibule is believed to result from peripheral sensitization of nerves to increased mechanical, and thermal stimuli, and pain-inducing substances. Allodynia can be measured with Quantitative Sensory Testing (with an algesiometer) of the vestibular mucosa, 214 although in the office setting, the Q-tip test and pain mapping are adequate for diagnosis if other findings are normal.
It is also well known that vulvodynia can be associated with numerous other chronic comorbid pain conditions:
- chronic fatigue syndrome
- temporomandibular joint syndrome
- endometriosis
- fibromyalgia
- interstitial cystitis
- irritable bowel syndrome
The most prevalent conditions are irritable bowel syndrome, interstitial cystitis, and fibromyalgia.215 216 217 218 Patients often suffer from other bodily pain besides the comorbidities and have lower pain thresholds in regions remote from the vestibule. A number of painful tender points remote from the vestibule have been identified that support this observation.219 Vulvar hypersensitivity and frequent comorbidity within the pelvis and remote areas, such as the temporomandibular area, accentuate the heterogeneity of vulvodynia from different neurophysiological mechanisms.
Chronic pain conditions including vulvodynia, fibromyalgia, interstitial cystitis, and irritable bowel syndrome are now known to be frequently underdiagnosed and are much more prevalent than previously estimated. Despite their disparate clinical presentations, studies of specific pain disorders report similarities in their findings such as systemic sensitivity, as illustrated with peripheral pain testing,220 similar alterations on functional MRI scanning of the pain network associated sites in the brain,221 and differences in descending pain modulation in the central nervous system.222
Also of importance is work on the sensory processing of vulvodynia by Hampson et al. who evaluated local (vulvar) and remote (thumb) pressure-evoked pain sensitivity in 24 LPV/PVD patients and 13 age-matched healthy individuals. As a positive control, they also measured thumb pressure pain in 24 fibromyalgia patients. Vulvodynia and fibromyalgia patients had similar insular brain activations which were greater than healthy controls in response to thumb stimulation. Vulvodynia patients vs. controls showed greater degrees of activation during thumb stimulation within the insula, dorsal midcingulate, posterior cingulate, and thalamus.223 This work also supports the embryological relationship between the urogenital sinus and the vaginal vestibule, where the urethra, vagina, and greater vestibular glands open. It has been hypothesized that the endoderm-derived tissue comprising the vestibule, urethra, hymen, and lower third of the vagina has special immunologically protective characteristics.224 225
Research studies: Development of central sensitization
(See detailed discussion of central sensitization in Pain Basics).
Central sensitization is a powerful idea, as it provides a physiological mechanism for sustained pain in the absence of the original, precipitating stimulus. Prolonged activation of peripheral nociceptors can trigger enhanced activity of spinal cord cephalad projecting neurons in proportion to intensity, repetition, and duration of the nociceptive input.226
Advanced technological functional magnetic resonance imaging (fMRI), performed during painful vestibular pressure, demonstrated similar activation in cerebral pain centers as in other chronic pain conditions. Grey matter density was increased in the pain regulation and stress-associated areas of the brain compared to controls.227
Group differences in touch and pain thresholds—and their neural correlates—were studied in women with provoked vestibulodynia (N = 15), and pain-free control women (N = 15). Results from quantitative sensory testing and self-report measures indicated that, as compared with controls, women with LPV/PVD exhibited allodynia (i.e. pain in response to a normally non-painful stimulus) and hyperalgesia (i.e. an increased response to a normally painful stimulus) at vulvar and non-vulvar sites. Also, brain imaging analyses revealed decreased difference scores between touch and pain in the S2 area in women with LPV/PVD compared with controls. These findings support previous findings of allodynia in women with LPV/PVD. There were no significant reductions in difference scores between touch and pain for regions related to cognitive and affective processing of painful stimuli. The results of this study contribute important information to the general pain and vulvodynia literature in elucidating the specific sensorimotor neural mechanisms that underlie hyperalgesia in a chronic pain population and have implications for differentiating neural processing of touch and pain for women with and without LPV/PVD.228 Taken together, these findings indicate that a unique feature of pain amplification may exist in LPV/PVD patients. This pain sensitivity may be attributed to either the chronicity of pain (central sensitization)229 or, alternatively, may be seen as a reflection of an intrinsic defect in mechanisms of pain regulation.230 Consideration of the contribution of central pain regulatory mechanisms may help to explain the variations in the clinical presentation and treatment outcomes in subgroups of LPV/PVD patients.231
Research studies: Autonomic dysfunction: postural hypotension
Vulvodynia can be accompanied by autonomic dysfunction, although its status as a cause or consequence of pain is not yet known.232 The dysfunction is also seen in other chronic pain syndromes. The autonomic dysfunction observed demonstrates higher resting pulse rates and lower systolic blood pressure in women with vulvodynia, compared with controls.233 Of further interest, women with primary vulvodynia have lower systolic blood pressure than women with secondary vulvodynia.234 Rodent data indicate that the density of autonomic nerve fibers can be influenced by the gonadal hormone milieu.235
FACTOR: Musculoskeletal effects
Introduction
The pelvic girdle is a major part of a kinetic chain, the lumbar-pelvis-hip complex comprising the abdomen and spine from above and the hips from below. Within it, the three layers of pelvic floor muscles (PFM) regulate posture, support the pelvic organs, provide lumbosacral and pelvic (trunk) mobility and stability, and support normal defecation, urination and enhancement of sexual pleasure. Basically, the function of the pelvis is influenced by the biomechanics and pathomechanics of the spine, sacrum, coccyx, and lower extremities.236
Research studies: Local and referred pelvic pain with superimposed pelvic floor muscle hypercontractility resulting in introital narrowing and muscle pain may arise with, cause, or be the result of musculoskeletal dysfunction.
Over the past two decades it has been recognized that LPV/PVD is frequently accompanied by varying degrees of pelvic floor muscle dysfunction. If there is muscle weakness (hypotonicity) resulting in organ prolapse or urinary incontinence not associated with vulvodynia, therapy to strengthen PFM is necessary to strengthen the muscles. But in a 2005 study, researchers reported that 90% of women with provoked vestibulodynia demonstrated hypertonic or overactive pelvic floor muscles.237 Other studies have confirmed that hypertonicity of PFM is associated with dyspareunia, vulvodynia and pelvic pain.238 239 240 242 It is likely that the muscle dysfunction present is maintained by the ongoing mechanical allodynia.243
FACTOR: Psychosocial influences
Introduction
There is widespread acknowledgement that pain of all kinds,244 including vulvodynia, develops or is supported via biopsychosocial etiological pathways.245 But, perceptions of vulvodynia still largely hold fast to the dualistic distinction: pain is either from the mind or from the body. There is currently little comprehension of how psychosocial factors may contribute to vulvodynia outcomes. While there are several studies on psychosocial factors in vulvodynia,246 247 and its negative impact is significantly associated with psychological, emotional and sexual difficulties,248 there is no coherent understanding of how psychosocial factors impact outcomes or interact with relevant disease variables.249
Research studies: somatization, depression, anxiety, and hypervigilance; history of early childhood abuse
A 2021 review revealed that vulvodynia presents both similar and unique cognitive, behavioral, and interpersonal features compared to other chronic pain conditions. But, in addition, negative sexual cues, body image-related factors during intercourse, partner factors, self‐efficacy beliefs, and penetration cognitions, in relation to pain and sexual functioning may play significant roles.250 The pain picture with its nociception, circuitry, signals, and neurotransmitters is not complete without the contributions from the higher centers of the brain: thoughts and emotions, memories, and interpersonal factors. Women with vulvodynia tell of significant feelings of isolation (“I am all alone”) and invalidation (“No one believes that I am in pain”).251 In the absence of visible pathology, women with vulvodynia receive little validation from health care providers and continue to hear “it’s all in your head,” even if this is not said aloud. This stigma is part of gender and ethnicity inequalities, resulting in poor treatment of their pain.252 Some women report feeling broken, inadequate, and worthless regarding the sexual components of their relationships. Guilt and shame add to their misery.253 254
Childhood maltreatment (CM) is a known factor leading to subsequent health problems.255 Disturbances in attachment security are related to childhood maltreatment.256 Attachment (ATT) develops throughout childhood based on the stability and security of the infant-caregiver relationship, and influences later adult romantic relationships. Attachment security is characterized by two dimensions: attachment related anxiety (negative representation of self, fear of abandonment) and avoidance (negative representation of “other,” discomfort with emotional intimacy).257 Couples with vulvodynia scoring high on attachment anxiety may exaggerate the threat value of pain,258 so that dealing with pain-related cognitions and emotions may prove to be more challenging for them.259
Those scoring high on attachment avoidance may minimize the threatening aspect of vulvodynia and employ cognitive and behavioral distancing strategies, such as denying distressing cognitions and emotions.260 Both ATT and CM are known as distal factors: events or conditions occurring a long time before the present problem, and both are associated with the occurrence, severity and adjustment to LPV/PVD. To date, there is no research related to ATT and CM connected to treatment efficacy for LPV/PVD; it is thought that ATT and CM may act as moderators in relation to different treatments. Women with more insecure attachment history (anxiety/avoidance) or a history of CM may benefit less from programs with higher interpersonal contexts, such as couples therapy or sexual therapy. In one study, 103 women with LPV/PVD were randomized to a 12 week treatment of either lidocaine or couple therapy to see if ATT and CM were predictors or moderators of sexual satisfaction, distress and function both post treatment and after 6 months. The validated testing instruments used were the Global Measure of Sexual Satisfaction, Female Sexual Distress Scale-Revised, and Female Sexual Function Index.
Both CM and ATT were moderators of treatment outcome: women in couple therapy with greater ATT avoidance had worse outcomes on sexual satisfaction and function compared with the lidocaine group. The authors emphasize the importance for clinicians to make note of and focus on distal factors such as CM and ATT when working with treatment plans for LPV/PVD, as these parameters may affect more interpersonal elements of treatment such as trust and compliance, and ultimately, treatment success.261
Intimacy, which includes empathic responses and self-disclosure, may act as a protective factor for women and couples coping with vulvodynia. Among 50 couples with vestibulodynia, both partners observed and reported that greater empathic responses were associated with better sexual satisfaction and lower sexual distress.262 Feeling understood, accepted, and cared for by a partner may promote better overall sexual and relationship adjustment, as well as quality of life in the face of challenges to couples’ sexuality such as vulvodynia.
It should be noted, however, that the overly solicitous partner who backs off from trying to engage in intimacy at the first expression of pain, may also cause negative results: avoidance of sexual interactions, increased pain perceptions, and feelings of isolation on the part of both partners.263 264
Pain catastrophizing (tendency to magnify, as well as feel hopeless and ruminate about the pain), attributions about the pain, and self-efficacy are three cognitive-affective disorders related to intra-individual and interpersonal associations with couples’ outcomes. Lower catastrophizing seems connected to lower pain levels.265 Partners’ higher negative pain attributions (“Elise and I are stuck with this pain problem forever and it is affecting our whole life”), were associated with their own greater psychological distress, poorer relationship and sexual satisfaction.266 A prospective study among women with vulvodynia revealed that increases in pain self-efficacy (the degree to which a woman believes she can manage the pain effectively), but not decreases in catastrophizing, were associated with lower pain.267 This information underscores the important role of cognitive-affective processes in women’s pain experience and the couples’ sexual, relational, and psychological adjustment.268
Some research has suggested that psychosocial factors could contribute to the onset and maintenance of chronic vulvar pain.269 270 This difference was not noted in other controlled studies.271 Several controlled studies from different clinical samples revealed that women with vulvar pain report higher depression scores than controls. These depressive symptoms, however, are not necessarily within the clinical range.272
What else is new and useful?
Animal models are being successfully used to “fast-track” understanding of vulvodynia.
Several in vivo models, primarily in rodents, have been developed to study vulvovaginal hypersensitivity, genital pain, and female sexual behavior. The models have supplied us with new information on the neural pathway neurotransmitters, and inflammatory mediators which are part of the peripheral and central mechanisms of genital pain and sexual function. Importantly, the models have demonstrated translational relevance to the clinical features of vulvodynia in women. In addition, P116 (a protein of previously unknown purpose) mainly produced by fibroblasts) has been recently identified to promote neuropathic pain in rodents providing potential for studies and possible interventions.273
The models focus on the different mechanisms thought to contribute to vulvodynia in women, including, at this time:
- Early life stress
- Repeated Candida infections
- Vaginal hyper-innervation
- Nociceptor sensitization induced by pro-inflammatory agents
- Mast cell mediated allergic responses related to vaginal hyperinnervation and tactile hypersensitivity
- Central sensitization from repeated pelvic and urogenital infections
In addition, there is an in vitro model using human vulvar fibroblasts, created with the goal of understanding the inflammatory pathways affected during LPV/PVD.274 275 276
The use of animal models is a basic step in the understanding of the fundamental mechanisms underlying the function of sensory systems as well as a step towards the development and proof of new possible strategies for their modulation. Working with receptor modulation involved in nociceptive input transmission, researchers can reproduce in rodents various painful states observed in humans. The models of vulvovaginal hypersensitivity might provide validated platforms for drug screening and drug development for vulvodynia in the future.
New information from the unfolding field of Multi Omics
Currently, our ability to turn out large scale molecular data and information is outpacing our ability to translate and generate clinically actionable approaches: our ability to analyze and interpret data to put it to good use in clinical practice.
Multi Omics is a new approach where the data sets of different “omic” groups are combined during analysis. For example, in the field of genomics, execution of novel sequencing techniques turns into cost-effective and rapid interpretation of an entire genome and the study of all genes at once, rather than gene by gene analysis. A wide array of additional omics technologies has been constructed, with “omics” indicating the comprehensive study of the roles, relationships, and actions of various types of molecules in cells of an organism. The main challenge will continue to be the development of methods for extracting useful information from the multidimensional and complex datasets to guide clinical practice.277
New information from Metabolomic Profiling
In a new area of hormonal study, information from untargeted metabolomic profiling of vaginal fluid and plasma in women with LPV/PVD and healthy women was combined with pain testing and brain imaging in women from each group. The hypothesis was that women with LPV/PVD would show differences in vaginal and plasma metabolites involved in steroid hormone biosynthesis compared to healthy women. Sensitivity analyses were also completed to determine the impact of hormonal contraceptive use on the study results. Women with LPV/PVD compared to healthy controls had significant reductions primarily in vaginal fluid concentrations of androgenic, pregnenolone, and progestin metabolites involved in steroidogenesis, suggesting localized rather than systemic effects in the vagina and vulvar vestibule. The observed diminutions in androgenic metabolite levels showed large effect size associations with increased vulvar vestibular pain and vulvar muscle tenderness, and decreases in androgenic and progestin metabolites were associated with decreased connectivity strength in primary sensorimotor cortices.278
Neuroimaging
Neuroimaging is being used in some studies to elucidate normal and abnormal pain pathways and changes in the brain particular to vulvodynia.
Structural and functional magnetic resonance imaging (MRI) studies have demonstrated many kinds of brain differences associated with the development of chronic pain.279 Few studies, however, have applied these techniques to study vulvodynia. In a project using pressure (perceived as painful in patients but not in controls) to the posterior portion of the vulvar vestibule as a stimulus in women with LPV/PVD and healthy controls, women with LPV/PVD showed significantly higher activation levels in the insular and frontal cortical regions of fMRI than did controls, mirroring activation patterns noted in other chronic pain conditions.280 With follow-up study, women with vulvodynia were compared to healthy controls and fibromyalgia patients (positive controls).281 Local (vulvar) and remote (thumb) pressure-evoked pain processing was assessed using fMRI. Both pain groups showed overlapping insular brain activations that were greater than in healthy controls during thumb stimulations. Significant differences between vulvodynia subgroups (primary/secondary, provoked/unprovoked) were observed in the posterior cingulate (thumb stimulus) and in the precuneus region (vulvar stimulation), suggesting heterogeneous neuropathologies. When whole-brain voxel-based morphometry was used, increased gray matter density was demonstrated in young women with LPV/PVD, increased gray matter correlating with increased pain.282 This discovery contrasts studies in patients with other chronic pain conditions documenting decreased gray matter density; experts speculate that gray matter density might increase in young pain patients and decrease in older individuals with long-standing pain.
The most common symptoms related to the vulva and vagina are itching, burning, pain, pain (or fear of pain) with touch or penetration, and odor or abnormal discharge. Itching tends to be more common with candidiasis or the vulvar dermatoses, whereas degrees of pain can be related to any of the conditions. Our Diagnostic Algorithm addresses the process of diagnosis for all of these symptoms, starting with the patient’s history (Anno B). After the history, specific symptoms and their locations are your next set of clues to diagnosis. One should not arrive at the diagnosis of vulvodynia, until completing each step of the diagnostic pathway. At the same time, the clinical diagnosis of LPV/PVD, GVD, or PN should be considered in all women (of any age or ethnicity) who present with symptoms of dyspareunia or vulvovaginal pain without identifiable cause.
All complaints should be collected using the “descriptors” listed above. Where is the discomfort located? Is it localized or diffuse? Is it intermittent or constant? Does it wax and wane? What circumstances influence its presence? Does it occur only with touch or is it “spontaneous,” independent of touch? For how long has it been experienced? In terms of pain with attempts at tampon use, insertional dyspareunia, or extreme discomfort with speculum exams, has this always been so (primary) or was penetration comfortable at one time but is now uncomfortable (secondary)?
Women with vulvodynia do not always use the words “pain” or “vulva” to describe what they are feeling. They often cannot describe location in words. (Even Eve Ensler’s well-known and lauded Vagina Monologues is written mainly about the vulva). Table K-4 and Table K-5 cover the range of terms used, and clues offered. Women are very specific about speculum use and its effects on them.
Table K-4. What does vulvodynia feel like? Women answer:
| “It feels like knives are there– little paring knives on good days, butcher knives on bad days.” |
| “ It feels like my pubic hair is caught, or something is pulling it out.” |
| “This feels like it should be red and on fire when you look at it.” |
| “The vulva and vagina feel swollen, dry, and painful– like sitting on a cactus.
They are sensitive to touch and pressure. Intercourse feels like a rope burn since I have major difficulty lubricating.” |
| “It feels like broken glass there. I can’t even sit.” |
| “Burn, burn, burn!” |
| “It’s raw. There’s an itch-burn sensation.” |
| “I”ve had this pain since I was a child, and it’s getting worse” |
These descriptors are common in GVD or PN, as well:
| “ It feels like a tennis ball wedged in the vagina.” |
| “I am constantly aware of the vulva/vagina. I can’t escape!” |
| “Sitting is impossible! I have to remove my clothing when I am at home and lie down in a bathrobe.” |
| “Sometimes the toilet seat is the only place I can sit.” |
Here is what a woman with vulvodynia tells her clinician about the pelvic exam and about sex.
Table K-5. Clues given about difficult pelvic exam or penetrative sex:
| “I have never been able to use tampons” |
| “You must use the smallest speculum.” |
| “These exams are painful; my doctor says I need to relax more.” |
| “ I am very small down there and it’s just too tight.” |
| “I can’t have sex at all.” |
Similarities in symptoms of vulvar pain without identifiable cause make diagnosis impossible without a careful exam. They are provoked by touch or spontaneous (not precipitated by touch), dull or sharp, stabbing, cutting, aching, or burning, stinging, with irritation and soreness, sometimes with an itch component, constant or sporadic, present for hours, days, weeks, then regressing for varying lengths of time, then rebuilding. Symptoms may be diffuse, without clear borders, or focal, or may change location; they may be bilateral or unilateral or include urinary symptoms with negative cultures. Symptoms may be exacerbated by touch or pressure, by sitting, biking, tight clothing, chafing, tampon insertion, or sexual touch or penetration. Intercourse may be feared, possible, tolerated, or impossible. There is often no pain in the absence of touch, although for some women, there can be a background of spontaneous (or unprovoked) discomfort that may be described as an “unpleasant awareness.”283
The pain may be mild, moderate, or severe, leading to various types of dysfunction: decreased mobility or ability to enjoy exercise or sports, inability to wear stylish clothing, depression, debilitation, inability to insert tampons, inability to experience pain-free sexual activity, feelings of inadequacy, hopelessness, fear, and desperation.
Primary and Secondary Vestibulodynia
The importance of whether pain with touch or attempt at penetration is primary (present from any first attempts at tampon insertion or penetration) or secondary (occurring after a period of comfortable penetration) has been studied.284
In 2010, Goetsch, et al found that women with primary vestibulodynia had significantly increased nerve fiber density in areas of pain than those with secondary vestibulodynia.285
In a 2011 study, Leclair and Goetsch re-examined archival vestibulectomy specimens from 88 premenopausal women with vestibulodynia to determine whether primary and secondary vestibulodynia represent different pathologic pathways. Records were reviewed and histologic sections were stained for hematoxylin and eosin to grade inflammation, S100 to highlight nerves, CD117 for mast cells, estrogen receptor alpha and progesterone receptor. 42 of 88 cases had primary vestibulodynia (48%), and 46 had secondary (52%). Use of combined contraceptives was about equal in younger (median 26.5 years) women with both primary (52%) and (median 26.5 years) secondary (54%) vestibulodynia.
Nulliparity was 83% in primary cases, 67% in secondary cases. Symptom duration was longer in primary (median 6 years) versus secondary (median 4 years). There was good agreement between pathologists evaluating histologic sections for inflammation, neural hypertrophy/hyperplasia, and hormone receptors. Significant neural hypertrophy and hyperplasia (p= 0.02) and increased progesterone receptor nuclear immunostaining (p=0.04) were noted in primary vestibulodynia compared with secondary vestibulodynia. Estrogen alpha expression was also increased in primary vestibulodynia when symptom diagnosis was less than 5 years.
They concluded that significantly different histological patterns existed in primary versus secondary vestibulodynia, suggesting separate biological mechanisms and possible distinct conditions. Primary disease shows increased progesterone receptor staining compared with secondary disease.286
A 2015 study found that patients with primary LPV/PVD had a longer duration of pain than those with secondary LPV/PVD, but no significant subtype differences in pain intensity during intercourse were identified. There were also no differences in sexual, psychological, or relational functioning between the two groups, both of whom had sexual dysfunction and impaired psychological functioning, leading to the conclusion that both groups needed the same therapeutic interventions.287
A 2018 study showed that there was no difference in pelvic floor muscle evaluation findings between those with primary and those with secondary vulvodynia, also confirming the need for the same interventions.288
The development and maintenance of primary and secondary LPV/PVD appear to involve different brain and brainstem pathways. In 2023, in comparing 105 nulliparous women ages 18-45 years with primary and secondary LPV/PVD with pain during intercourse of at least 5/10 numerical rating score (NRS) and healthy controls (no details given), researchers began to study the default mode network (DMN) of the brain and brainstem, a set of regions in the brain thought to be more active during passive (resting state) tasks than tasks demanding focused external attention. Areas of the brain included in the default mode network include the medial temporal lobe, the medial prefrontal cortex, and the posterior cingulate cortex, as well as the ventral precuneus and parts of the parietal cortex. Women with secondary LPV/PVD have increased dorsal attention–somatomotor network connectivity, (a network of cortical regions that support sustained attention and working memory), whereas women with primary LPV/PVD predominantly show increased intrinsic resting state connectivity within the brainstem and the default mode network. The significance of these findings indicates that women with secondary LPV/PVD demonstrate more evidence for central amplification of sensory signals, while the brain circuitry of women with primary LPV/PVD shows alterations responsible for the processing and modulations of ascending and descending peripheral signals. Women with secondary LPV/PVD, when compared with women with primary LPV/PVD, also reported greater incidence of early life sexual abuse, greater pain catastrophizing, greater 24-hour symptom unpleasantness, and less sexual satisfaction.289
Evidence of these differing central mechanisms operating in primary and secondary LPV/PVD represents the potential for development of effective therapeutic approaches for women with LPV/PVD. The demonstrated brain alterations imply that women with primary LPV/PVD may respond more to centrally targeted therapies, such as cognitive behavioral therapy, hypnosis, and mindfulness along with centrally targeted medications, while women with secondary LPV/PVD may respond better to therapies targeted at reducing increased peripheral nociceptive signaling.290
Before the initial exam of any patient with vulvar pain, ask yourself: Can the patient tolerate examination at this point? A woman with LPV/PVD, in particular, is frightened to have any kind of exam. Signals that the exam may be difficult are important (Tables K-4 and K-5). Spoken or written clues in a careful history will point to vulnerabilities such as comorbid chronic pain or chronic stressors (anxiety, depression, past trauma, etc). Past sexual abuse is especially difficult for a woman to reveal and discuss. Fear of any kind of penetration may be overpowering, whatever the patient’s background. It will take continued development of a trusting relationship to obtain an ability to proceed to exam. Who among us has not thought, “It will be okay. I am a kind and gentle person. I can talk her through this,” and has not been dismayed by an outcry at a very light touch and an inability to reassure or continue. Separating the visits into 1) history for the first visit, and 2) examination at the second may work to help alleviate the fear and may be necessary before an adequate examination can be achieved. A contract established early between patient and clinician that the exam will be stopped any time the woman asks, is reassuring. See Annotation D for more on this.
Abdominal palpation may provide information correlating with urinary (painful bladder syndrome) and other problems. See Annotation C for Targeted Non-genital Exam. Some clinicians look for umbilical tenderness on examination as it has been found in some patients with primary vestibulodynia. In these cases, it is postulated that umbilical hypersensitivity represents a congenital defect in urogenital sinus-derived tissue, mirroring pain in the endoderm-derived epithelium of the vestibule, possibly pointing to an embryological or immunological reason for the pain.291 The understanding that the vestibule and the lower third of the vagina all stem from the embryological urogenital sinus endoderm has begun to generate new studies that indicate that the area presents a unique immune profile, differing from that of other mucosal sites.292 293 294 This concept is explored in the section on Pathophysiology: Studies related to biomedical and psychosocial factors associated with vulvodynia, above.
Inspection of the vulva alone (in the case of a patient not allowing touch) will allow the clinician to evaluate the normalcy of the anatomical structures and the color, texture, and integrity of the vulvar skin as a first step to ruling out “known” causes of discomfort. If necessary, the patient herself may be able to spread the labia to expose structures and skin if she is too afraid of being touched by another.
Vulvar examination for evaluation of pain
As similar as many complaints of vulvovaginal pain are, there are differences between the subsets that will lead you to diagnosis. First, ask yourself: what will I be touching and how? Each vulvar area to be evaluated for pain is in close proximity to normal tissue and each small area may provide a path to diagnosis. Performing the exam systematically and with care to observe the patient’s reaction to touch at each step will help to isolate your first diagnosis. In each case, there may be no physical manifestation for the pain: no skin color or texture changes, no lumps or bumps. Now, at the time of exam, the patient can point to where she feels her symptoms.
Localized provoked (and/or spontaneous or mixed) vulvodynia/vestibulodynia (LPV/PVD)
If one compares LPV/PVD to any other pain condition, few to none present with the same remarkably uniform clinical findings, particularly with respect to marked mechanical allodynia, located immediately adjacent to pain-free skin and mucosa.295 The vestibule (and/or peri-clitoral area in the rare case of clitorodynia), is tender, sometimes exquisitely so, with gentle Q-tip touch inside Hart’s line, often adjacent to the hymenal ring. Fleeting erythema with Q-tip touch may be seen, but is not consistently present. The tenderness may extend around the introitus or be localized to the posterior edge between 5 and 7 o’clock. (Some clinicians specify 4-8:00). Pain elicited in the vestibule with light mechanical touch (allodynia) in the setting of no other identifiable disorder, present for three months or more, is the current definition of LPV/PVD. Some researchers are suggesting that there may be differences or subgroups in patients with insertional dyspareunia, one group with pain at the vestibule alone, another with pain in the hymenal ring itself, another with pain in the pelvic floor muscles, in some cases all three being present, possibly related to different initiating causes.296 Farmer, et al, are also studying different aspects of female genital sensation, looking not only at vestibular pain but pain associated with feelings of fullness (distension) in the vagina as possibly being part of a subtype of vulvodynia. They propose that there may be two types of pain sensation going on, somatic (in the vestibule) and visceral (in the vagina).297
It remains to be seen, through research, if further subgroups of vulvodynia will be identified. See this diagram for the differences between somatic and visceral pain:
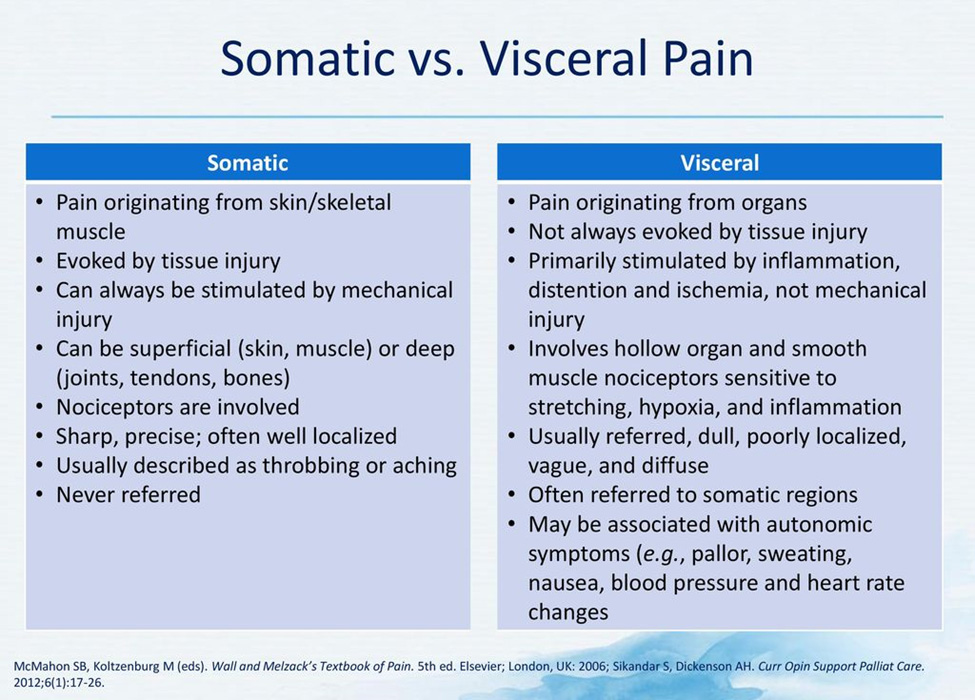
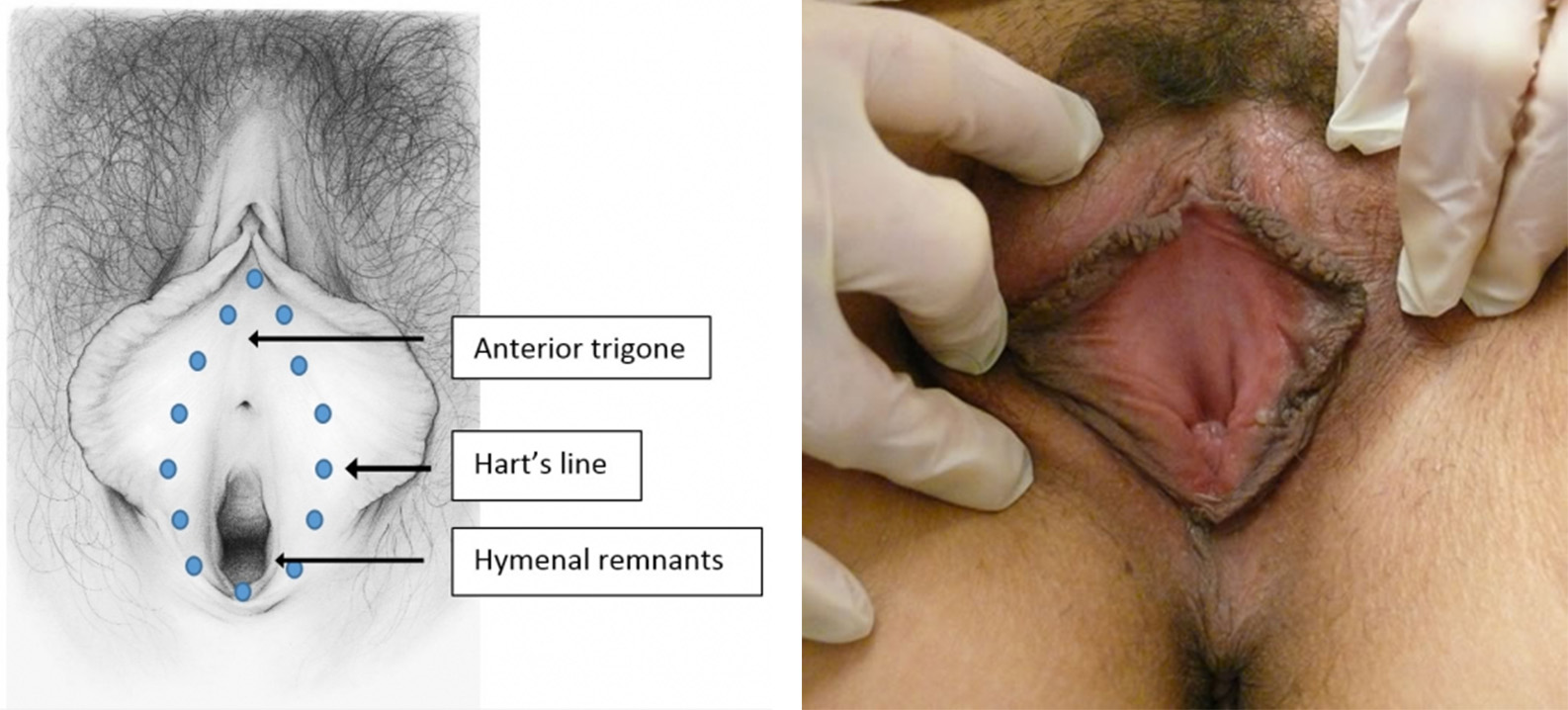
The vestibule is inside of Hart’s line. (drawing by Dawn Danby from The V Book by Elizabeth G. Stewart, MD)
Q-tip test: Use of a Q-tip (thin, cotton (or dacron) tipped applicator) is helpful in pain mapping with light touch, starting in areas that are not painful.298 299 The use of a numerical rating pain scale (0-10),300 301 along with Q-tip touch, helps to identify location and intensity of the pain and is also useful in comparing, from one visit to another, whether or not improvement is taking place. The Q-tip, used very gently with patient permission, also allows for movement of vulvar structures for better visualization.(See Anno I for more on this). Some experts do question this subjective evaluation as an adequate outcome measure for assessment, diagnosis, or treatment of vulvodynia,302 and some researchers are using vulvalgesiometers303 to better quantify pain sensations. However, the cotton swab applicator (Q-tip test) is still used in both clinical and research settings. There is a question about whether the Q-tip test can adequately mirror the pain felt during attempts at penetration. For this reason, some researchers use the “tampon test,” to study efficacy of treatment.304 A recent study, however, found that the tampon test does not actually represent the patient’s pain with sexual penetration adequately either.305 In some studies, the vulvalgesiometer is being used to test pain via sensitivity to pressure in the vaginal tissue as well as to test for pain in the vestibule.306 307 One small study comparing mucosal thickness by transvaginal ultrasound between women with LPV/PVD, those with untreated genitourinary syndrome of menopause (GSM), and healthy controls found that patients with either GSM or LPV/PVD had similar thinning of the mucosa and similar degrees of pain.308 The clinical or diagnostic significance of this finding is unknown.
Generalized vulvodynia and pudendal neuralgia
Generalized vulvodynia (GVD) may be located anywhere on the vulva, as is the case with pudendal neuralgia (PN), where pain occurs over the sensory distribution of the pudendal nerve: in the vagina, vulva, labia, and clitoris. Symptoms of pain and paresthesia may extend as far as the mons, groin, inner leg, buttocks, and abdomen. Pain from PN is often unilateral. Constipation, (often exacerbating pain after bowel movement), 309 urinary frequency, urgency or hesitancy,310 and vulvar, perineal, or rectal pain during or after intercourse,311 are all associated with both GVD and PN.
If the patient’s discomfort is generalized, (outside of the vestibule or in multiple places throughout the vulva) or unilateral, the Q-tip can be used to gently trace the course of the pudendal nerve, looking for allodynia, dysesthesia, or hyper or hypoalgesia. Clinicians familiar with assessing for PN may also use a pin prick test in the same way. Abnormal sensation with pin prick testing along the pudendal nerve may confirm the diagnosis of PN. Another test that is used by physicians experienced in evaluating for pudendal neuralgia is pudendal nerve block. If the nerve block is successful in eliminating the pain, a problem in the pudendal nerve is confirmed.312 Evaluation of the pudendal nerve may also include a 0-10 pain scale: https://www.pudendalhope.info/node/18 This detailed description of degrees of pain, written by someone who suffered from PN, may be helpful to anyone experiencing pain or trying to help patients with pain.
For information on testing for PN, not routinely done by gynecologists or dermatologists, please see: https://www.pudendalhope.info/node/69.
Nantes criteria for pudendal nerve pain include (1) Pain in the anatomical territory of the pudendal nerve. (2) Worsened by sitting. (3) The patient is not woken at night by the pain. (4) No objective sensory loss on clinical examination. (5) Improvement with anesthetic pudendal nerve block.313 PN may also be present without all of these criteria being met. In his 2021 chapter in Female Sexual Pain Disorders second edition, Marvel states “it is likely that many (if not the majority) of women with generalized or unprovoked vulvodynia have pudendal neuralgia because, in my experience, most”…of these women…”have altered sensation in the pudendal nerve distribution when tested clinically.” 314
These specific (Nantes) criteria may or may not be present for generalized vulvodynia, for which some researchers report sudden onset and symptoms worsening at night. PN and GVD are not consistently associated with painful sex.315 Some researchers believe that localized provoked vulvodynia and generalized provoked vulvodynia may be the same condition on a time spectrum, rather than two different clinical entities.316 317
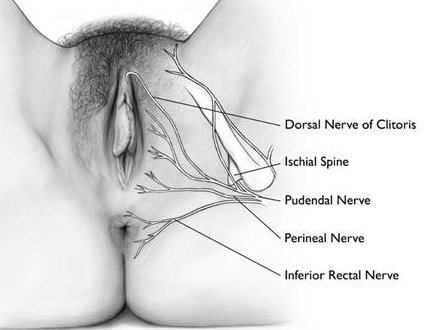
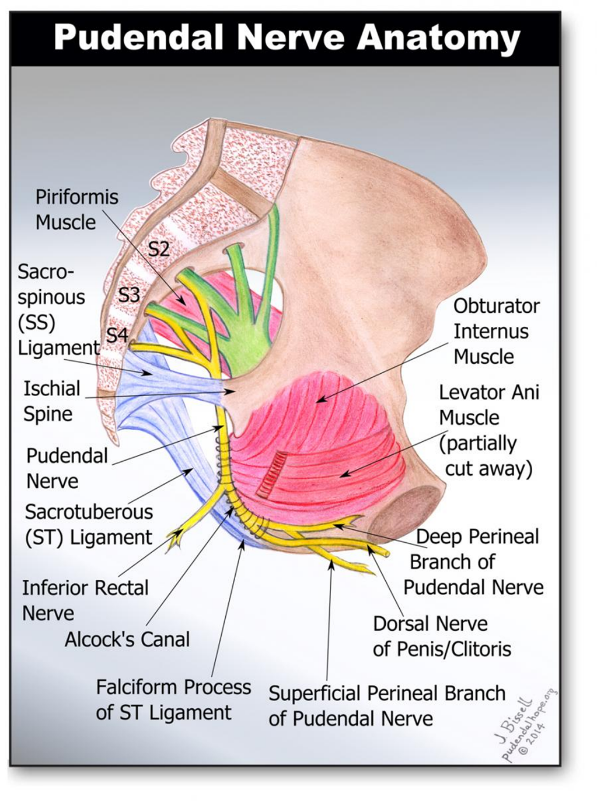
First drawing by Dawn Danby from The V Book by EG Stewart and Pudendal Nerve Anatomy drawing by J. Bissell from https://www.pudendalhope.info/node/13
Digital exam of the hymenal ring: After the Q-tip test (which should have been applied to all the non-painful and painful areas in question, including the hymenal ring), the next step of the exam includes gentle palpation of the tissue, including the hymenal ring (hymenal remnants), if allowed by the patient. A lubricated finger is placed on the hymenal ring (with minimal touch to the vestibule and without touching the vaginal musculature) to determine any abnormalities in the hymen, thickening, strictures, or pain with pressure or distension (gentle stretching). Palpation and gentle stretching with two examining fingers is comfortable in non-virginal women without a vulvovaginal complaint. Lev-Sagie, et al have pointed out that there may be a different subgroup of patients whose complaint of dyspareunia is accompanied by pain in the hymenal ring rather than the vestibule.318
Evaluation of the pelvic floor musculature: The clinician then stands to evaluate the pelvic floor muscles, this maneuver preceding speculum exam. Some studies are questioning the physiological source of vestibular pain, suggesting that even mucosal pain may originate in the pelvic floor muscles.319 320
In LPV/PVD, the pelvic floor muscles are typically tense and tender with gentle but firm pressure with a single digit applied within the vagina, laterally and posteriorly to the introitus, then deeper in the vagina to evaluate the levator ani. Pain may also be present in the absence of palpable pelvic floor muscle hypertonicity. When asked to contract and then relax the pelvic floor muscles, patients with vulvodynia may not be able to do so.321 (Exam of the pelvic floor is described in Annotation L). With PN, a constant element on physical examination is a replication or worsening of the pain during rectal or vaginal digital pressure in the area of the ischial spine.322 Although this may occur in an asymptomatic woman, unilateral tenderness in this location suggests PN by the Nantes criteria.323
Speculum examination can cause significant pain. Use of the smallest speculum (even pediatric size), slowly and gently rotated, is necessary, if tolerated by the patient, since a view of the four quadrants of the vaginal mucosa is helpful to assure normal anatomy and absence of abnormal secretions. (Annotation M: https://vulvovaginaldisorders.org/annotation/annotation-m/ )
Laboratory testing
The exam must be completed by performing vaginal pH, KOH, whiff test, and saline wet prep microscopy, obtaining yeast culture, Pap and HPV testing if pertinent, STI testing, visual evaluation of the cervix, and, if possible, bimanual exam. If the patient can not tolerate insertion of a finger or a speculum, she may be able to accept insertion of a Q-tip for the purposes of evaluating vaginal secretions. In some cases, we have collected secretions that have appeared at the introitus, with no insertion into the vagina at all, in order to perform pH and microscopy.
Biopsy is not routinely indicated to diagnose LPV/PVD, GVD, or PN, although in at least one study, previously unidentified “known” disorders such as HPV-related LSIL, lichen simplex chronicus, or lichen planus were found on histological examination of vestibulectomy specimens. 324
See https://www.pudendalhope.info/node/69 for information on diagnosis of PN. Clinical neurophysiology tests have low diagnostic efficacy in the diagnosis of PN and must be considered to be only complementary investigations. Nerve response time that is slower than normal (<2.2 ms) by pudendal nerve motor latency testing (PNMLT) may indicate nerve damage but is not considered a highly sensitive test.325 PNMLT tests only motor function and is only significant if abnormal; the sensory nerve may be compressed or damaged and this test will not reveal that. Currently no mechanical modality exists for sensory testing of the pudendal nerve. Pudendal nerve blocks can be useful for both diagnosis and treatment of PN. See https://www.pudendalhope.info/node/11 for information on pudendal nerve blocks.
MRI is rarely used for diagnosis of vulvodynia or PN, but may be helpful in cases of intractable pain, to ensure that there are no tumors or other types of obstruction causing pain, no spinal pathology, or Tarlov cysts. (https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Tarlov-Cyst)
The exam findings of vulvodynia can be summarized as: a normal vulvar appearance (with or without mild erythema) and normal vaginal walls and secretions in the presence of pain with touch.326 Investigation should be directed at confirming or denying other pathologies such as Candidiasis, dermatosis, atrophy, etc. The presence of dermatological changes such as lichenification, excoriation, ulcerations, or masses suggests an alternative or concomitant diagnosis that needs further addressing. There are no other specific tests for vulvodynia or for pudendal neuralgia.
A full work up (as described in the steps of the Diagnostic Algorithm) is necessary to determine if the location of symptoms (Annotation I: Pain and symptom mapping and the Q-tip test) and their descriptors can be identified as any of the conditions inTable K-1and is therefore pain related to a specific disorder. In the case of LPV/PVD, GVD, or PN, there will be no other physical findings, (other than possible erythema in the vestibule). The combination of the history, the pain map showing distribution, and the absence of findings that suggest a known disorder (this may require more than one visit), leads to the diagnosis of spontaneous or provoked vulvodynia (GVD,LPV/PVD), or pudendal neuralgia (PN). (Vaginismus is covered in https://vulvovaginaldisorders.org/pelvic-floor-dysfunction/)
Any of the characteristics in the table below may be related to any type of vulvodynia. To facilitate diagnosis, we have attempted to sort indicators most strongly associated with the subtypes. We have added a column particular to pelvic floor dysfunction/vaginismus which is covered in Pelvic floor dysfunction and vaginismus to highlight some differences in presentation between vulvodynia, pudendal nerve pain, and vaginismus.
Table K-6. Non-exclusive list of exam findings characteristic of three subsets of vulvodynia plus pelvic floor dysfunction/vaginismus (PFD/V)
| LPV/PVD | GVD | PN | PFD/V | |
| Fear of pain with touch/penetration | X | X | ||
| Pain with penetration or inability to tolerate penetration | X | X | ||
| Exquisite pain in the vestibule alone, spontaneous or provoked | X | |||
| Posterior or circumferential vestibular tenderness | X | X | ||
| Pain in the hymenal ring/remnants | X | X | ||
| Vulvar pain anywhere outside of the vestibule, localized or diffuse | X | X | ||
| Pain over sensory distribution of pudendal nerve | X ? | X | ||
| Unilateral vulvar pain | X | X | ||
| Unilateral vestibular pain | X | X | ||
| Presence of umbilical sensitivity | X | |||
| Worsened by sitting | X | X | ||
| Awakened by pain at night | X | |||
| Sudden onset | X | |||
| Replication of pain with vaginal or rectal digital pressure on or near ischial spine | X | |||
| Correlated with urinary or bowel-related pain | X | X | ||
| Pain during or after penetrative sex | X | X | X | X |
| Hypertonicity in the pelvic floor muscles | X | X | ||
| Improvement with pudendal nerve block | X | |||
| Impairs sexual relationships | X | X |
Remember that there may be more than one problem. If a dermatosis or infection is found, additional pain or symptoms in another area may suggest vulvodynia as well as infection or dermatosis. For example, a woman with multiple, pruritic syringomas of the labia majora also has spontaneous burning in the vestibule from vulvodynia. Whether the two problems are related is still unclear.
Vulvodynia is not a single disease but a diverse group of disorders with possible subsets and sub groups yielding the same symptoms, regionally located in the same anatomical site of the vulva and vagina. It is a disease of the central nervous system with altered central neural sensitization resulting from amplified or persistent synaptic transmission, and altered ascending or descending pain modulation.327
Introduction
Vulvar pain from a known disorder often resolves with treatment or control of the condition, but in some cases, treatment of the pain is necessary in addition to treatment of the disorder. In addition, the location of either vulvar pain of known cause or vulvodynia impairs sexuality and feelings of normalcy.
Treatment of vulvodynia has suffered from lack of knowledge about its etiology. The absence of identifiable physiologic pathology in cases of vulvodynia has led to the view that the pain may be solely due to psychological factors. This position is as erroneous as emphasizing only the physiological aspects of the conditions.328
The Classic Cartesian (dualistic) Approach
This dualistic, “either/or” (mind or body), way of assessing complaints of pain with no visible evidence of disease (often referred to as functional somatic syndromes (FSS) or complex chronic pain conditions (CCPC) and including irritable bowel, fibromyalgia, chronic fatigue/myalgic encephalomyelitis, TMJ dysfunction, headache, painful bladder syndrome, and vulvodynia)329 has long persisted in the way clinicians have been trained to evaluate patients.
Homeostasis and Vulvodynia
The work of Cannon in 1932 with his coining of the term “homeostasis,” 330 and Selye’s studies on stress and stressors in the 1940’s and 50’s 331 332 promoted development of a more holistic, biopsychosocial model of evaluation and care in the 1970’s and 80’s, a concept that supports treatment options for vulvodynia and other chronic pain conditions today.333 334 336 337 338 339 The complexity of vulvodynia on the physiological, psychosexual, and social levels can be seen as a challenge to homeostasis that must be addressed on multiple levels at once.
The simple definition of homeostasis is a “state of balance among all the body systems needed for the body to survive and function correctly.” Another definition: a dynamic, self-regulating, negative and positive feedback process by which biological systems maintain stability while adjusting to changing external (and internal) conditions.340 Implicit in the concept is dynamism (also called allostasis), where fluctuating stimuli (including stressors) can challenge physiological equilibrium in both positive and negative ways. The adaptation to these stimuli within many systems at the same time also provides redundancy, that is, backup systems that accommodate to the threat of disease. Disease is the disruption of homeostatic mechanisms, the overriding of allostasis/homeostasis. The following illustration is from Lu, Wei, et al. 341
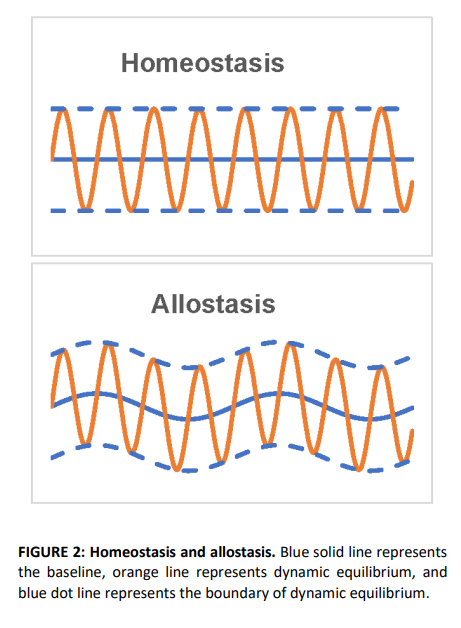
As Marvel says, “Persistent pain has no benefit or purpose; it is a dysfunction in the system.”342
Maladaptation in the attempt to regulate homeostasis may also lead, in the case of chronic pain, to central sensitization, now believed to be a factor in vulvodynia and other chronic pain conditions. The mechanisms of central sensitization mirror the impact of overwhelming stress on homeostasis. (See Pain Basics, Central Sensitization).
Difficulties with the Recent Treatment Approaches
Medical treatment strategies for vulvodynia have long been based on what is known about treatment of other chronic pain conditions and have followed expert opinion and a “trial and error” approach. Randomized controlled trials have been rare and poorly powered and medical treatments numerous, using multiple approaches and combinations. 343 344 345In a prospective study of 282 patients enrolled in the National Vulvodynia Registry who received care from experienced vulvovaginal specialists, 78 different treatments were identified and categorized by type and number: topical (85%), physical therapy (52%), and oral medications (45%), with 73% of patients receiving two or more treatments. Six months after treatment, women reported improvements in the following categories: well-being, general pain, pain with intercourse, catastrophizing, and anxiety, as well as in physical, emotional, and social functioning, but all showed worsened sexual functioning (except for the improved pain).346 Both the overall improvement in symptoms despite a multitude of treatments, and the overall worsened sexual functioning, call into question exactly what works and what does not, and why.
The Game Changer
In 2010, Foster, et al, did one of the few randomized, carefully controlled trials looking at the most common treatment modalities of the day. Their study showed that oral desipramine and topical lidocaine, as monotherapy or in combination, failed to reduce vulvodynia pain more than placebo, calling into question the belief systems of most specialists. They concluded that “placebo or placebo-independent effects are behind the substantial pain improvement seen in all treatment allocations.”347 Those insights led to a cascade of re-evaluations of current therapies and a burgeoning of Foster’s own thoughts on the subject.
Current Recommended Therapies
Treatments for the management of LPV/PVD now focus less on medications and more on the treatments that have evidence of success: psychosocial interventions, pelvic floor physical therapy, and vestibulectomy. While surgery has been considered the option of last resort, many clinicians, in the absence of proven medical therapies, have turned to vestibulectomy sooner than “last resort,” if psychosocial and pelvic floor PT therapies do not help enough over time.348 349
These three major treatment modalities are discussed below following Behavioral and Educational Interventions and prior to a review of medical treatments once commonly in use.
Basic behavioral and educational interventions
- Identify and eliminate any possible pain triggers
Stop offending irritant activities on the vulva (Annotation J: Lifestyle issues). Although soap is an unlikely culprit, every aspect of the patient’s lifestyle must be explored for possible contributing factors. Inquire about use of detergents, perfumes, deodorant sprays, wet wipes, and other feminine hygiene products, tight clothes, underwear worn to bed, sitting for long periods, bicycle or horseback riding, etc. and advise on best behaviors. (See General Vulvar Care handout).
- Diagnose and suppress any vaginitis: candida, inflammatory vaginitis, trichomonas (Annotation P: Vaginal secretions, pH, microscopy, and cultures). Remember that bacterial vaginosis is not a significant cause of pain.
- Suppress herpes if active. (Centers for disease Control:www.cdc.gov/std/herpes/treatment.htm)
- Marsupialize recurrent/persistent Bartholin cysts (Atlas of Vulvar Disorders). We have seen provoked pain in the vestibule chronically inflamed by such cysts.
- Provide adequate local estrogen if there is atrophy. (Annotation P: GSM) Diagnose and treat lesions of dermatitis, dermatosis. (Atlas of Vulvar Disorders)
- Recognize systemic disease such as drug reaction, Sjögren, or Crohn disease, and treat vulvar manifestations. (Annotation O: The vaginal epithelium)
- Consider seminal plasma allergy if history suggests. (Semen allergy: Atlas of vulvar disorders)
- Consider issues such as painful bladder syndrome (interstitial cystitis), pelvic floor dysfunction. (Annotation L: The pelvic floor)
- Refer to physiatrist or physical therapist to evaluate and treat musculoskeletal problems possibly affecting pelvic floor and pudendal nerve. (Annotation L: The pelvic floor.)
- Consult experts regarding pain in other areas, (e.g., gynecologist for endometriosis, urologist for interstitial cystitis), to make sure that all components of a regional pain syndrome are addressed.
- Always ask yourself if there is any possibility of a premalignant or malignant lesion. (Atlas of Vulvar Disorders.)
2. Educate about vulvar pain
Explain normal vulvovaginal anatomy and functionality as necessary. Use a mirror during your exam. Use handouts (to patient handouts), website referrals, and local support groups. Emphasize that pain may need to be managed, not cured. Describe the typical slow and gradual regression of pain that may occur. Discuss global impact on every aspect of sexuality (Vulvar pain and sexuality), emphasizing that pain must be managed first; then work on sexual function can occur. Discuss the psychology of pain, factors that worsen pain, and the importance of taking charge. Learn about a woman’s erroneous cognitions about her pain, and work to eradicate them. Discuss fear avoidance techniques. Suggest Margaret Caudill’s How to Manage Pain Before It Manages You, 3rd edition. New York, Guilford Press, 2009 or The Pain Management Workbook by Rachel Zoffness, New Harbinger Publications, Inc. Oakland, CA, 2020. Healing Painful Sex: a woman’s guide to confronting, diagnosing, and treating sexual pain. Deborah Coady, Nancy Fish. Offer counseling for support, anxiety, depression, or relationship problems.
3. Provide information about comfort measures
Flares of pain can be minimized in a number of ways (Self Help Tips for Vulvar Skin Care: http://www.nva.org). By far, the most important comfort measure is the technique of “Soak and Seal:” sitting in comfortable water (tub, sitz bath under the toilet seat, or gentle hand held shower for 5-10 minutes twice daily). A woman who is physically unable to use the tub or sitz bath may protect the bed with plastic sheeting and use sopping wet warm compresses. Pat dry gently; then seal in the moisture with a film of petrolatum or mineral oil. Some find pure coconut oil soothing and non-irritating. Cool gel packs wrapped in a soft cloth are also important to pain management. Although ice against the skin can cause frostbite, pain is significantly allayed by cold packs.
4. Set expectations
While the symptoms of vulvodynia may remit entirely, it is possible that they improve but do not disappear; i.e. they are managed, not cured. This is an important concept to emphasize at the initiation of therapy. There is no crystal clear cure, no magic bullet, no one-size-fits-all approach. There is improvement with time and effort. The preceding statement is particularly difficult for a woman who has “tried everything.” However, exploration with her may reveal some gaps: poor provider-client communication and “chemistry,” inadequate trial time, underuse of all aspects of a therapy, e.g., lack of internal work with physical therapy, insufficient time with cognitive behavioral therapy, unrevealed history of sexual abuse.
5. Provide guidelines for sex
We ask women who have pain on penetration to stop vaginal intercourse until there has been some improvement in symptoms. Ongoing intercourse in the presence of pain is a negative reinforcer and can lead to secondary vaginismus. (Annotation L: pelvic floor dysfunction/vaginismus) We encourage open communication between a woman and her partner about her pain with the effort to prevent feelings of rejection. We encourage intimacy and the pursuit of any mutually agreed upon pain-free alternatives to vaginal intercourse.
If sexual intercourse is possible with a level of comfort acceptable to the patient, we recommend use of a lubricant such as a few drops of plain baby oil without fragrance; this may be well tolerated as long as condoms are not being used. Latex condoms are not compatible with oil-based lubricants or medications. In women using condoms, a water-based lubricant is appropriate (e.g., an iso-osmotic, pH balanced product, e.g., Pre-Seed®). Some women find a decrease in sexual pain with use of side-lying or rear entry. See Guidelines for sex when you are having pain. (See lubricants) See section on Lidocaine in Medical Approach below for possible use to reduce fear of intromission pain.
The following circular model of provoked vestibulodynia by Rosemary Basson illustrates the compounding effects of ongoing chronic pain and/or sexual dysfunction on the patient’s sense of well being. Emotional distress associated with premorbid anxiety, depression, catastrophization, harm avoidance, hyper-vigilance, low self esteem, and a tendency to perfectionism may be associated with neuroplastic changes in the central nervous system leading to central sensitization and pain amplification. Feelings of being sexually substandard compound the etiological factors and lessen sexual motivation and response. Pain-induced cognitive changes may impair processing of sexual stimuli generally and at the time of sexual activity. Motivational changes associated with chronic pain circuitry may further impair sexual motivation. Stress responses of muscles and skin add to the pathophysiology. 350 A book that directly addresses chronic vulvovaginal pain and sexuality is The Pleasure Prescription: A surprising approach to healing sexual pain, by pelvic floor physical therapist Dee Hartman and sex therapist Elizabeth Wood.
Basson’s circular model of provoked vestibulodynia351
Psychosocial Intervention
The Fourth International Consultation on Sexual Medicine, in its consensus-based guidelines, recommends both physical therapy to the pelvic floor and psychosocial modalities for first line treatment of vulvodynia.352 Chisari, et al, on reviewing the literature on the impact of psychosocial factors on the incidence of vulvovaginal pain and sexual outcomes, also promotes a psychosocial approach to conceptualizing and managing vulvodynia.353
Approaches include cognitive behavioral therapy (CBT), medical pain management if necessary,354 sex therapy, and psycho-education, 355 offered either individually or in combination.
Psycho-education involves conveying information to the patient about the ways in which our mental outlook colors our perceptions, experiences, relationships, and day to day health. In the case of vulvodynia, the patient might be made aware of characteristics such as negativity, hopelessness, hypervigilance, catastrophizing, fear avoidance, self-stigmatizing, feelings of shame, isolation, and other emotions and thoughts and how they impact pain and coping strategies.
CBT is a talk therapy based on the following core principles:
- Psychological issues are partly based on problematic or unhelpful patterns of thinking.
- Psychological issues are partly based on learned patterns of unhelpful behavior.
- Psychological issues are partly based on problematic core beliefs, including central ideas about yourself and the world.
- People experiencing psychological issues can learn better ways of coping with them. This can help relieve their symptoms and improve their mental and emotional health.356
This approach requires understanding about and acceptance of the power of thought and emotion in relationship to the experience of pain. It often includes the concept of mindfulness, which is the practice of bringing attention to the present moment without judgment, in an attempt to disentangle the present moment (which is “not the end of the world”) from worries and pressures associated with fear, rumination, and catastrophizing, all of which can contribute to ongoing pain. Patients are taught to reframe their experiences to reduce fear and stress.
CBT, pain management, and sex therapy aim to reduce pain and improve women’s and partners’ sexual functioning, sexual well-being, and relationship satisfaction by targeting the thoughts, emotions, behaviors, and couple interactions associated with vulvodynia. These modalities benefit by focusing on multiple dimensions of vulvodynia beyond the pain itself, including sexual relationships and psychological stress.357
The next step aims to move women and their partners towards more adaptive emotion co-regulation358 and communication during a period of reconnecting through non-sexual physical and emotional intimacy. In this way, their sexual repertoire is redirected away from intercourse/penetrative sex alone. In its place, non-threatening, pleasurable touch may lead to shared sensations of desire, arousal, and sexual intimacy of a type that is acceptable to both partners.359
Three different randomized studies of women with LPV/PVD have evaluated CBT.
1) Women with LPV/PVD were randomized to CBT, electromyography (EMG) biofeedback, or vestibulectomy. All three had reductions in pain at six months, with vestibulectomy showing the best results. The CBT group reported significant improvement in pain at a 6-month follow-up, and had similar pain levels during intercourse as women who received vestibulectomy at the 2.5 year follow-up, both reduced compared with pre-treatment. Women in the EMG/biofeedback group responded as well as those in the CBT arm at the six month follow-up, but not at the 2.5 year follow-up.360 361
2) Another study randomized women with LPV/PVD to receive either a topical corticosteroid or group CBT for a period of 13 weeks. Women who received group CBT reported significantly higher treatment satisfaction, less severe pain and pain catastrophizing, and better global improvement in pain and sexuality than the group who received the topical corticosteroid.362 The authors concluded that group CBT resulted in changes on more domains of LPV/PVD than topical corticosteroids.
3) A third randomized trial found that individual CBT yielded significantly greater improvements in pain and sexual function than individual supportive psychotherapy; these improvements were maintained at the one year follow-up point.363
Additional studies offer evidence that CBT is an empirically validated non-invasive treatment that can be administered in different formats to women with vulvodynia.364 365 366 367
While the literature supports the use of psychological, sexual, and behavioral therapy to treat vulvodynia symptoms, it is important to remember that these therapies may not address the underlying peripheral disease mechanism.368 369
Pelvic Floor Physical Therapy
Whether pelvic floor muscle dysfunctions are a consequence or a cause of genital pain remains unknown.370 Hypersensitivity and tenderness of the pelvic floor muscles are usually present in women with vulvodynia, related to shortening of the muscles, inflammation, altered perfusion, and neural contributions. Pelvic floor physical therapy (PFPT) aims to rehabilitate the pelvic floor musculature by enhancing muscle proprioception, relaxation, discrimination and elasticity, normalizing muscle tone, desensitizing painful vulvar tissue, and providing secondary reduction of fear of pain and vaginal penetration. 371 Physical Therapy is discussed in detail in Annotation L: The pelvic floor.
PFPT includes several methods that can be used singly or in combination. Most frequently employed are
- Education regarding avoidance of irritants, tight clothes, perfumed products, and discussion of sexual function at the time of each visit.
- Electromyography (EMG) biofeedback: an EMG vaginal sensor is inserted in the woman’s vagina for her to retrain her pelvic floor musculature using feedback on a video screen about the strength of her vaginal muscle contractions and relaxations.
- Manual therapy: the physiotherapist uses hands-on stretching and massaging of the pelvic floor to enable muscle relaxation and tissue mobility.
- Electrotherapy: to help a woman increase muscle proprioception, a low-voltage electrical current is used.
- Dilators with insertion techniques: to desensitize the vulva and vagina and reduce the fear of pain and vaginal penetration, a woman is taught to use vaginal dilators (“trainers”) that gradually increase in size during in-office sessions and home exercises. These are not used to enlarge the introitus, but rather, to engender confidence about the patient’s capacity for penetration.372
Myofascial release
Myofascial pain can occur whenever muscles have contracted repetitively or over long periods of time. It may be associated with “trigger points:” tender “knots” or tight bands in muscles that can be palpated by a physical therapist or physiatrist which are very sensitive to touch and can reproduce the patient’s symptoms with both local and referred pain responses. They can be associated with autonomic symptoms, such as sweating, lacrimation, flushing, vasomotor reactions, and temperature changes.373 These trigger points can be linked to stress or anxiety which may cause unconscious muscle tightening. Myofascial release is a type of low load, long duration stretch massage used by physical therapists to relax the muscles and allow them to elongate to a more normal position.374 375 A 2019 study showed that myofascial physical therapy (MPT) “has anatomical, neurophysiological, and psychological therapeutic effects alongside long-lasting pelvic pain alleviation. Specifically, MPT works on relieving hypertonicity, reduces the sensitivity to experimental pain, improves the functionality of the endogenous inhibitory system, and decreases psychological distress (i.e., state and trait anxiety levels, pain catastrophizing, somatization, and depressive symptoms). These systemic effects position MPT as a multisystemic therapeutic intervention for patients with chronic pelvic pain syndrome (CPPS),” including vulvodynia/dyspareunia.376 The authors of this study hypothesize that the close, trusting relationship that develops between the women’s health physical therapist and the patient plays a strong role in the positive results achieved through PFPT.
Trigger point injections
Aside from myofascial release massage, noninvasive measures for treatment of trigger points include “spray and stretch,” transcutaneous electrical stimulation, and other physical therapy techniques.377 (“Spray and stretch,” NOT used in treatment of vulvodynia, refers to spraying the skin with an instant topical anesthetic refrigerant and then applying stretch techniques). Invasive treatment of trigger points includes injections, in the case of vulvodynia usually performed by a physiatrist, anesthesiologist, neurologist, or trained gynecologist, with local anesthetics such as lidocaine or bupivicaine, corticosteroids, botulinum toxin, plain saline, or by dry needling. The mechanism of action of trigger point injections is thought to be disruption of the knots or bands, especially their abnormal contractile elements, by the mechanical effect of the needle or the chemical effect of the agents injected, resulting in relaxation and lengthening of the muscle fibers.378 Studies are looking into the use of ultrasound in locating painful trigger points and guiding placement of needles.379 380
An extensive review of PFPT for LPV/PVD found consistent effectiveness of PFPT across studies, with significant improvement in pain in 71-80% of women.381 As a predictor of treatment outcome, only more severe pre-treatment pain was associated with higher pain severity at a two to five year follow-up checkpoint for EMG biofeedback.This study compared CBT, biofeedback, and vestibulectomy, not PFPT.382 Just one randomized clinical trial has looked at the efficacy of PFPT, which was compared to overnight topical lidocaine treatment in 212 women with PVD. In this study, women who received PFPT had a significantly larger improvement in pain intensity during intercourse, sexual function, and distress from pre-treatment to post-treatment compared to those who used lidocaine. The beneficial effects of PFPT were maintained at the 6-month follow-up. 383 All of these findings yield strong evidence that physical therapy should be a first-line treatment for LPV/PVD.384
Combination therapies
Sex therapy, physical therapy, dietary advice: Major improvements in women’s sexual function and pain were reported by two published uncontrolled quantitative studies evaluating a multimodal approach to the treatment of genital pain. Multimodal treatments integrate sex therapy and physical therapy in a nonstandardized manner, such that not all participants received the same combination and duration of interventions. A retrospective qualitative study of 29 women with vulvodynia who participated in such a treatment program suggested that 27 reported a significant benefit, with 9 being pain free.385 This program consisted of psychotherapy, physical therapy, and dietary advice.
CBT, physical therapy, regular follow up visits: Another study using the same design was conducted in 19 women with vulvodynia who participated in a multimodal treatment that involved group CBT, physical therapy, and regular medical appointments. Results indicated that participants reported increased knowledge and tools to manage the pain, improved psychological well-being, a sense of validation and support, and an enhanced sense of empowerment.386
Somatocognitive therapy: A physiotherapy practice in Norway describes their model of utilizing a biopsychosocial (BPS) approach that includes somatocognitive therapy (SCT) in the treatment of women with LPV/PVD, an approach that encapsulates and puts into action current thinking.387 Kaarbøm et al, state that “SCT is a complex physiotherapy intervention that addresses physical, psychological, and psychosexual aspects of PVD with a multimodal approach in order to improve sexual function and reduce vulvar pain.” Their list of strategies includes the following: 1) therapeutic alliance, 2) bodily exploration (on the part of the patient), 3) pain education, 4) coping with thoughts and emotions (referred to by some as “emotion regulation”), and 5) structured homework. These techniques, all provided from within the physiotherapy practice, are reviewed in the article. In the United States, for the most part, care is provided by separate specialists: the appropriate physicians and other clinicians, physical therapists, sex therapists, psychologists, psychopharmacologists, who all must communicate with each other for the best outcome.
Vestibulectomy
Vestibulectomy represents an invasive procedure, but is an option if conservative treatments such as medications, CBT, and physical therapy to the pelvic floor fail to help the pain and restore more comfortable sexual intercourse.388
History of perineoplasty/vestibulectomy
In 1981, Woodruff used a modification of a perineoplasty he had described earlier in a textbook. 389 The surgery treated 42 women ages 21-29 with strictures or fissures of the posterior fourchette and dyspareunia unresponsive to medical management (18), severe itching (9), or outlet obstruction from extensive condylomata (15). The procedure consisted of the excision of a “ triangular portion of perineal skin with its base at the vaginal outlet and apex near the anus. 3-4 cm of the vaginal mucosa were undermined to form a flap attached just above the anus to cover the triangular portion that was removed. The painful or scarred tissue under the flap was excised and the freed vaginal mucosa was then approximated to the skin edges with interrupted Vicryl suture. The patients with itching also received alcohol injections of the perineum. Biopsies from the severe itching group were positive for lichen sclerosus; one of the condylomata cases biopsied positive for carcinoma-in-situ. Patients were followed for 6 months to one year. All recovered well and were free of dyspareunia. The itching resolved with testosterone treatment of the lichen sclerosus. Condylomata/CIS were controlled with 5-Fluorouracil. He reported 100% success for all the women.390
In 1983 Woodruff further modified the perineoplasty procedure for treatment of “infected vestibular glands” in 15 women, ages 22-39 years, all of whom had been sexually active and initially without dyspareunia. No other patient details are given. On examination, all had “tiny erythematous foci immediately lateral and circumferentially involving the hymenal ring. The ring itself was often constricted to a significant degree and often tender to palpation.”
Woodruff’s new version was the “same as that described as perineoplasty, with the inclusion of the hymenal ring and adjacent 0.5 cm of tissue. The incision extends from approximately 0.5 cm beneath the urethra to the fourchette internally with a parallel incision 0.5 cm lateral and usually terminated just above the anal orifice. If the labia minora encompassed the entire outlet to the fourchette, the lower 1 to 2 cm of the labia may be included in the excised tissue. Both incisions may be included in the excised tissue. Both incisions may be extended in an anterior direction if the involved tissue extends to the periurethral portion of the vestibule …’’ “The vaginal epithelium is undermined for a distance of 2 to 3 cm from the fourchette, and the scarred subepithelial tissue is excised to prevent subsequent constriction. The vaginal epithelium is then exteriorized and sutured to the skin with polyglycolic acid (Dexon) or Vicryl. Histopathologic study has routinely demonstrated small glandular structures lined with transitional epithelium with adjacent mucus secreting acini.” Again he reported complete success.391
Perineoplasty is a surgical reconstruction procedure performed electively to correct tissue altered by vaginal delivery, episiotomy, or disease (lichen sclerosus), with a vaginal advancement to provide a cushion for the thrust of vaginal penetration.392
Vestibuloplasty includes excision of a localized painful area such as the posterior vestibule, usually with a vaginal advancement flap.393
Vestibuloplasty may also refer to the surgical undermining of the vestibular mucosa at the distal perimeter of the vulvar vestibule, removing only the submucosal minor vestibular glands, and closing without vaginal advancement.394
Simplified vestibulectomy was developed by Goetsch, a simple procedure with minimal removal of tissue. With a skinning technique, every tender part of the vestibule inside Hart’s line is excised. The majority of the hymen is left intact to be used in the closure to cover the denuded area.395
Complete vulvar vestibulectomy with vaginal advancement (also called complete vestibulectomy) involves excision of the vulvar vestibule from 10-2 o’clock posteriorly, including the mucosa adjacent to the urethra, the hymenal ring, dissection, and creation of an approximately 3 cm vaginal advancement flap, and a two layer closure to cover the defect of the posterior vestibule.
Modified (also called posterior) vestibulectomy was described in the early 1990s when Bornstein reported performing the procedure. It involved excision of the hymenal ring from the 3-9 o’clock position and of the superficial vestibular mucosa to Hart’s line.396 The vaginal advancement extended only half way down the perineum.397
Difficulties in comparing vestibulectomy procedures
These variations in technique of vestibulectomy are not always documented in the record, making it difficult to compare studies. Each surgical technique is often described by the same name. Changes in the technique may be used, even in the same series of study patients. Criteria for patient selection for surgery are not given. Use of controls has not been studied. The outcome criteria for “successful surgery” are often vague; standard terminology to assess success has not been developed.398 Time to follow-up may not be mentioned, or may be irregular, even within the same series. Follow-up may be only a few weeks or months, making difficult the determination of the rate of recurrence of vestibulodynia after surgery.399 Follow up is often done by postcard or telephone, so that examination is not performed. Pain or patient satisfaction are used as primary outcome measures and do not evaluate sexual function or quality of life.
In 2010, Tommola reviewed 33 journal articles evaluating surgical treatment for vulvar vestibulitis syndrome from 1996-2007. With the exception of Bergeron’s RCT, all were case studies without controls. In the evaluation of 17 of the 33 cases reviewed, surgical success was defined only by the patients’ self report on improvement of dyspareunia, reduction, or alleviation of pain. Overall, 91 ”were satisfied with the outcome.“ The visual analog scale (VAS) for dyspareunia decreased from a median of 9 to 3 (66.7%); (p<0.001). Posterior vestibular tenderness was absent in 34 patients (64.2%). Six (8.6%) patients developed postoperative bleeding and 11 (15.7%) mild wound infection. Bartholin’s cysts occurred in four (5.7%) patients. The author indicated that although surgery relieves pain in most patients, other treatment modalities are often needed. From all the studies reviewed, Tommola’s conclusion is that surgery seems effective and safe, with manageable minor complications and without sequelae. No straightforward recommendation of the best technique can be made.400
Conclusions regarding vestibulectomy
A 2020 review by Das, et al of modified vestibulectomy studies from 2009-2016 included 22 patients, ages 22-65 years. Most of the women were premenopausal (57%), sexually active (68%), and partnered (76%). Postoperatively, 17 of 18 women (94%) reported improvement. Pelvic floor physical therapy, medications, and lubricants were used both preoperatively and postoperatively.
Preoperatively, 21 (96%) patients reported dyspareunia, 17 (77%) reported burning, and 10 (46%) reported itching with pelvic examination, findings significant for point tenderness in 21(96%), erythema present in 13 (59%), and levator tightness or tenderness present in 15 (68)%. Nine of 22 patients were reported as having provoked vestibulodynia, one was reported to have focal vestibulodynia, and 14 were reported as having vestibulitis or vestibulodynia.
Qualitative analysis was achieved with 14 interviews conducted after the surgery from November 2018 to January 2019, 37-104 months after surgery. Of 14 participants interviewed, 13 (93%) reported improvement of pain after surgery, 11 (79%) reported satisfaction with surgery, 8 (57%) reported satisfaction with sexual function, and 11 (79%) reported recommending the surgery to others. The following lead themes were identified: vulvodynia symptoms significantly affect quality of life; there is difficulty and delay in diagnosis owing to lack of information and awareness among patients and health care providers; and surgical success and satisfaction are influenced by patient perceptions, with sexual dysfunction often persisting despite vulvar pain improvement.
In the follow-up performed in the study of vestibulectomy, 93% of interview participants described pain improvement and 79% spoke of surgical satisfaction.The researchers reported these results as consistent with high surgical success rates of 40-80% as compared with success rates of 40-80% with non-surgical interventions. They indicated, however, that only half of the participants reported sexual satisfaction post operatively, indicating that improvement in vulvar pain does not always translate to improved sexual function. The authors concluded that vulvodynia patients report improvement in pain and high overall satisfaction after modified vestibulectomy, but more variable long-term effects on sexual function.401
With this complexity, a systematic review of the success rates and complication rates of the different versions of vestibulectomy procedure is not possible.The only RCT of surgery for LPV/PVD by Bergeron et al in 2001, showed the greatest short-term pain reduction after vestibulectomy, compared with EMG biofeedback or group CBT. But at the 2.5 year follow-up, no difference in pain with intercourse was found in the surgery group versus the CBT group.402 In a review of vulvodynia 19 years later the same author said, “It appears that the variations in procedures for vestibulectomy are of minor importance and that surgery often improves symptoms.“ 403
Finally, in 2022 Bohm-Starke et al initiated a review of existing randomized controlled trials and non-randomized studies for pharmacological, physical therapy, psychosocial, surgical, and mixed interventions for LPV/PVD. They experienced an inability to compare study results due to heterogeneity in interventions and outcome measures and a low certainty of the evidence produced. They concluded that the review underlines the need for more methodologically stringent trials on interventions for LPV/PVD, particularly for multimodal treatment approaches. For future research there is a demand for joint core outcome sets.404
The 16% failure rate of vestibulectomy in Tommola’s review, with no relief or worsening dyspareunia warrants consideration. If there is failure with the last resort, misery continues for a significant number of women and their partners. Residual tender foci are possible, such as a hymenal remnant. Poor subject selection is one explanation; more treatment of the pelvic floor muscles and work with dilators could be helpful. Work with postoperative biofeedback has also brought improvement. Further work with cognitive behavioral therapy is another source of improvement.
There is also exploration of new possibilities. Maybe there is more to be learned from more detailed study of the vestibule, hymen and vagina. Farmer et al. have raised the question of vaginal distention with allodynia as sources of dyspareunia.405 Insertional dyspareunia may occur with constriction of the hymenal ring and anterior vestibular pain.406 Characterization of the nerves innervating the vulvar vestibule needs to be done.407
In recent years, the unique systems of vulvar inflammation and immunology in relationship to vulvodynia have been brought to light. Now, the exciting prospect of inhibiting these proinflammatory systems is beginning as therapeutic immune system blockers are being developed.408 409 These may help women who have not benefited from vestibulectomy, or may even prevent the need for surgery.
Approaches to vulvar pain using topical, intravaginal, and oral medications have been used in the treatment of LPV/PVD and GVD for many years, both as monotherapy and in combination.410 Evidence for efficacy has been variable and inconclusive, hampered by the lack of randomized controlled trials. Improvement has occurred but is thought to be related to the relationship with a knowledgeable, supportive practitioner, education, behavioral changes, improvement in mental outlook, physical therapy, and placebo effect. Even if pain is diminished, sexual function often lags behind.
In 2020, Bergeron, et al. published an excellent article synthesizing all that was known up to that point about etiology, pathophysiology, associated factors, and treatments for vulvodynia. 411
We include their table on treatments here, with permission from the publishers.
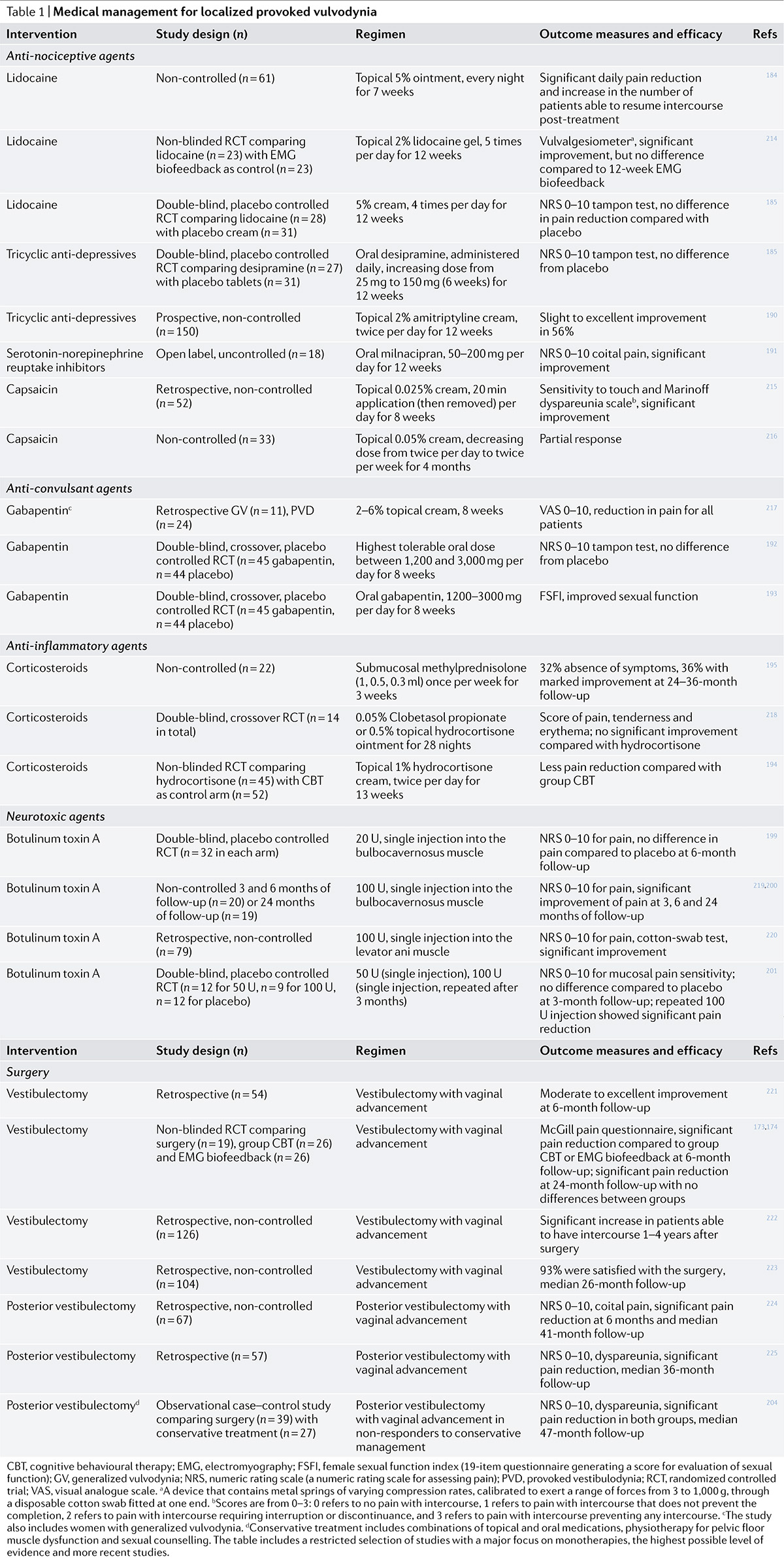
Medical approaches, continued:
Anti-nociceptive medications
Lidocaine/EMLA (eutectic mixture of local anesthetics, including lidocaine): Topical local anesthetics achieve their analgesic properties by blockading the sodium channels on peripheral nociceptors and preventing transmission of discharge signals from peripheral sensory nerves. With general acceptance that sensitization of peripheral nerves in the vestibule is the mechanism of the pain in LPV/PVD, sustained desensitization of these nerves through the application of topical numbing agents was a logical step.
Topical lidocaine 2-5% has been used in various anatomical locations and scheduling formats in women with LPV/PVD to try to reduce peripheral sensitization and reduce pain. An overnight application of 5% lidocaine was tested and found useful in 2003. 412 In the sole RCT comparing topical lidocaine to PFPT, however, use of lidocaine failed to demonstrate adequate benefit in alleviating dyspareunia.413 Most non-controlled trials showed an efficacy in the range of only 50%. Lidocaine is not recommended for long-term management or as a therapeutic agent. EMLA is most often used in the office prior to a painful procedure like vulvar biopsy.
Lidocaine used on a daily basis to “calm the nerve endings” in conditions of chronic vestibular pain is no longer recommended. However, practical use of topical lidocaine still has its place in treatment. For instance, application of lidocaine prior to planned and desired penetrative sex (or prior to speculum exams, outside of the initial exam) can alleviate fear of pain on penetration. 5% lidocaine ointment can burn intensely before it numbs the skin, but will provide excellent temporary pain relief. 4% aqueous lidocaine applied with a cotton ball prior to penetration may help, with less stinging from the medication. Even 2% lidocaine gel, applied with a cotton ball will numb the skin well. Ten to 20 minute application of one of these formulations prior to attempted penetration is most helpful. The ointment or gel can be wiped off and replaced with lubricant prior to interaction with a partner.
Capsaicin: The discovery of increased vanilloid receptor innervation (VRI) in women with vulvodynia and the agonist effects of capsaicin on vanilloid receptors located in the peripheral terminals of nociceptors sparked the use of capsaicin as a treatment. Capsaicin causes hyperesthesia on initial exposure, then sustained desensitization of burning and pain.414 The efficacy of capsaicin cream in LPV/PVD was evaluated through a prospective and a retrospective study, both of which claimed improvement in symptoms. However, lidocaine was used prior to capsaicin application to prevent burning or irritation, making discernment of the true effect of capsaicin impossible. 415 416
A recent warning has been issued by one dermatologist experienced in treating vulvodynia who states that the irritation caused by capsaicin is significant enough to avoid its use in most cases. 417 At this time, capsaicin is reserved for use only if standard treatment modalities have failed.
Anti-inflammatory Agents
According to patients’ experiences, oral non-steroidal anti-inflammatory agents such as ibuprofen are not helpful with vulvodynia.418
Corticosteroids
Since high levels of interleukin-beta have been reported in the hymenal region of the vestibule of women with LPV/PVD, and corticosteroids decrease the production of IL-beta, trials of corticosteroids were an obvious agent to try in the treatment of vulvodynia. Data indicate that topical corticosteroids are minimally effective in treating LPV/PVD,419 420 although intramucosal methylprednisone combined with lidocaine showed some symptom improvement in one small uncontrolled study.421
Interferon (INF): This injectable agent is approved for the treatment of condylomata acuminata (genital warts). Its method of acting is through downregulation of the expression of proinflammatory cytokines422 which are reported to be significantly high in the hymenal region of the vestibule of women with LPV/PVD. INF is also a potent inhibitor of mast cells, which may play a role in the initiation of LPV/PVD.423 Efficacy studies of INF in LPV/PVD have found a modest improvement with submucosal vestibular INF injections, but the agent is not currently recommended as a first line treatment for LPV/PVD.424.
Other anti-inflammatory agents
A cream containing the lysate of fetal fibroblasts as well as a combination of anti-inflammatory cytokines yielded a significant although modest decrease in vulvar sensitivity and intercourse pain compared with placebo.425 A low molecular weight heparin-enoxaparin with anti-heparinase properties for the treatment of LPV/PVD was used in a double-blinded, randomized placebo-controlled trial for the treatment of LPV/PVD. The enoxaparin treated women showed a greater decrease in vestibular sensitivity at the end of treatment and 3 months later, and 75% reported a decrease of pain greater than 20% compared with 27.8 % of the placebo group.426 These encouraging reports from small RCTs need further evaluation.
Neuromodulating medications
Antidepressants
Tricyclic antidepressants (TCA) have been the first-line treatments for neuropathic pain conditions for many years.427 For the precise analgesic mechanism, a suggested action may ensue from repeated stimulations of Beta-adrenoceptors by increased levels of noradrenaline in the synaptic cleft.428 Amitriptyline is often used for generalized unprovoked vulvodynia in clinical practice, and has been associated with favorable outcomes regarding pain in retrospective and non-controlled trials.429 430
However, RCTs lack strong evidence for TCAs regarding efficacy for LPV/PVD, although amitriptyline has been frequently used. Low-dose desipramine, assessed in a RCT of women with LPV/PVD, found no superior effect of desipramine compared with placebo.431 432 Topical 2% amitriptyline has been used to some success in one prospective study without a placebo-using control group. All the patients had either provoked or spontaneous vestibulodynia or both and had failed oral amitriptyline treatment. Positive response rate was 56% but only 10% of patients had complete pain relief and were able to have pain-free intercourse.433
Side effects of oral TCAs include weight gain, fatigue, tachycardia, and seizures.
TCAs are no longer recommended for the management of LPV. Other types of antidepressants used for neuropathic pain have not yet shown proven efficacy for vulvodynia.
Anticonvulsants
Gabapentin and pregabalin are well known anticonvulsants used to treat multiple chronic pain conditions and have been used for vulvodynia, as well. Both agents are believed to affect voltage-gated sodium channel function at nerve terminals, dampening depolarization and the release of pain-promoting neurotransmitters such as Substance P and glutamate.434 Gabapentin has been found to be helpful in the treatment of generalized, unprovoked vulvodynia.435 One double-blind crossover RTC yielded no difference in pain with the tampon test in women who received gabapentin compared with the placebo group. A second RCT, however, used the same dosage of gabapentin compared with placebo and found improved sexual function.436 While some women with vulvodynia report symptom relief with the use of various neuromodulating agents, larger trials are needed for better analysis.437
Hormones (this material is also in Pathophysiology: review of studies)
Role of estradiol and testosterone levels and oral contraceptives: A polymorphism in the androgen receptor significantly raises the risk of developing combined hormonal contraceptive (CHC )-induced LPV/PVD.438 CHC formulated with an estrogen and a progesterone lead to a lower serum estradiol and free testosterone by decreasing ovarian production of estrogen and total testosterone and by inducing the liver to produce increased levels of sex hormone binding globulin (SHBG). In addition, some CHCs contain synthetic progestins that act as testosterone antagonists at the androgen receptor.439 Besides this, it has been shown that CHCs induce morphologic changes in the vestibular mucosa, raising its vulnerability to mechanical strain.440 In addition, CHC use has been associated with decreases in mechanical pain thresholds441 and with decreasing clitoral size, labial thickness, and introital diameter. All of these reductions led to decreased orgasm, lessened sexual frequency, limited lubrication, and increased dyspareunia associated with CHCs.442
It follows, with all the effects on sex steroids, that researchers have looked for a connection between use of CHCs and development of LPV/PVD. Women who used CHCs before the age of 17 years had a relative risk of 11 of developing PVD.443 Both Bouchard, et al.444 and Harlow, et al. confirmed, in case-controlled studies, that early CHC use significantly increases the risk of developing LPV/PVD.445 In one study, use of CHCs containing ethinyl estradiol, even at a dose of <20 mcg, significantly increased the risk of LPV/PVD.446 There is also a non-controlled case series of 50 women who developed LPV/PVD while on CHCs; treatment with topical estradiol 0.01% and testosterone 0.1% was successful after cessation of the CHC.447 In 2019, Casado-Espada, et al,448 after an extensive review of the literature, published an excellent, comprehensive article about hormonal contraceptives and female sexual dysfunction. They point out that human sexuality is so complex that it is almost impossible to identify causes and effects of such complaints. Although combined hormonal contraception has been available since 1960, with a huge number of hormonal variations and devices invented to address efficacy for contraception and both positive and negative effects, there remains a dearth of randomized controlled trials in this field. And, there is some conflicting evidence about the role of CHC, with one population-based study of 906 women showing no increase in risk of vulvodynia.449
Pudendal and other nerve blocks and local anesthetic infiltration methods
Pudendal, spinal, and other nerve blocks, as well as local anesthetic infiltration approaches, have been used for diagnosis and management of generalized vulvodynia, suspected pudendal neuralgia, and even provoked vestibulodynia. Some researchers are asking whether management of the pudendal nerve is enough in cases of prolonged or severe vulvodynia and are trialing progressive blocks that affect nerves outside of the pudendal branches. Since the autonomic sympathetic nervous system conveys nociceptive messages from viscera to the brain, interventions with the sympathetic system are being used for perineal and vestibular pain management to some extent. Ganglion impar blocks, with lidocaine and triamcinolone injection around the terminal branch of the sympathetic chain in the presacral space, have been performed with good results for generalized vulvodynia in a small case series.450 However, this study only included four patients with previous treatment-failure vulvodynia that had been present for from six months to ten years. Hypogastric plexus and L2 lumbar sympathetic blocks are also being investigated. A good article that describes all the sympathetic ganglia types of blocks is that of Gunuz and Kenis-Coskun from 2017.451
In 2008, an observational study including 27 women concluded that serial multilevel nerve blocks (combination of caudal, epidural, pudendal, and local infiltration) significantly improved vestibular pain. Follow-up, however, was limited to 8 to 12 weeks and randomized placebo controlled trials were recommended.452
In 2012, the same group conducted a prospective, non-controlled pilot study to assess the efficacy of treatment using caudal, epidural, pudendal nerve block, and vulvar infiltration of local anesthetic agents. Thirty-two women with vulvodynia met criteria and 26 women completed the trial of five treatment sessions with multilevel local anesthetic nerve blockade with follow-up 2-3 months later. Vulvar pain significantly improved with this treatment, but there were no changes in sexual functioning.453
Another prospective observational non-placebo controlled pilot study was implemented by Weinschenk, et al in 2021. In this study, 162 consecutive patients with complaints of vulvar pain were evaluated, with 63 meeting criteria for vulvodynia. Of these, 45 ultimately completed the study, 36 with primary and 9 with secondary vulvodynia. Average age was 44.5 (+14.9). Vulvodynia had been experienced on average five years and all the patients had failed numerous other treatments. Many of these treatments were continued during the trial and some of the patients had concomitant known conditions such as lichen sclerosus, recurrent herpes simplex, or painful bladder syndrome. Success was identified as a drop in the vestibular 0-10 pain scale to < 4. The first three sessions consisted of bilateral perineal pudendal nerve blockades. If a drop in pain did not occur, nerve blocks were extended to other areas: the genitofemoral nerve via the inguinal channel, the hypogastric plexus via the vagina, and other areas depending on comorbidities such as painful bladder syndrome. Although there were many problems with this study, the investigators claimed 80% of the patients achieved a statistical reduction in pain to an average level of < 4 that persisted at a > 6 month visit.454
Serial therapeutic nerve blocks with local anesthetic and corticosteroid agents have shown success in some patients. Whether through the action of down regulation of sodium channel reactiveness on primary afferent nerves, disruption of tight connective tissue bands, or changes in proinflammatory substances, the serial nerve blocks seem to downregulate pathways that cause neurogenic pain. 455
Local anesthetic infiltration:
In a case series of five patients, Procaine 0.5% with hydrochloric acid (Procaine Serra) (0.5-1 mL) was injected subcutaneously into the painful areas of the vulva either once or twice, with complete or incomplete pain relief lasting for 7-18 months, allowing return to sexual functioning and tampon use in all the patients. This pilot study served as a test case for the concept of using locally injected anesthetic agents in this way.456
Pudendal nerve blocks (PNB) are both diagnostic and therapeutic. For suspected generalized vulvodynia or pudendal neuralgia, a properly placed PNB must reduce pain by at least 50% to be diagnostic. (Although nerve injury may be identified, it may not explain the perceived pain).457 Pudendal blocks may provide relief for the lower vagina, posterior perineum, and vulva, all supplied by the ventral rami of the second to fourth sacral nerve via the pudendal nerve for both motor and sensory innervation.458 A pudendal block will not provide pain relief for the anterior perineum, upper vagina, or cervix.459
Aside from subjective relief of pain after PNB, another diagnostic method is that of using pinprick testing along the pudendal nerve before and two hours after treatment; results in the men studied showed correlation between reduced pinprick sensation and reduced perceived pain.460
There have been attempts to identify the best approach for performing PNB. Ultrasound guidance has the advantage of not needing CT or fluoroscopy.461 One study showed no statistical differences in success rates when using either the finger-guided transvaginal technique or an ultrasound-guided transgluteal approach.462 There are many caveats relating to the ability of the initial PNB to diagnose PN. The patient should be referred to an experienced clinician for PNB and for treatment if PN or pudendal entrapment is suspected.
If injury to the nerve is demonstrated through PNB, treatment always starts with behavioral modifications that include avoidance of activities that repetitively compress or stretch the nerve and commencement of prescribed exercise like walking or running on a flat surface and swimming, use of a pudendal nerve protecting cushion, and a standing desk. Pelvic floor physical therapy may be helpful if the pelvic floor is affected.463
Therapeutic PNB is considered conservative treatment and helpful if it reduces pain, even if it requires repeating at (usually one-month) intervals.464 In a first report of using PNB for treatment of PN, a group of 26 women received a series of five CT-guided blocks at the ischial spine over a five month period, yielding a 62% reduction in pain.465 In a later prospective study of 55 patients, 87% had good to excellent outcomes466 In a 2009 study by Fannuci et al., 27 patients with pudendal neuralgia underwent CT guided pudendal nerve blocks. At one year follow-up, 92% showed continued clinical improvement.467 Pain relief that lasts past the PNB procedure(s) indicates that central downregulation is taking place. A randomized, double-blind controlled trial was carried out by Labat, et al in 2017, evaluating whether or not there was benefit in adding a corticosteroid to the anesthetic used in PNB.468 They found no statistically significant benefit to adding the steroid although corticosteroids in addition to an anesthetic agent are commonly used. There is no consensus about the use of steroids.469
For spontaneous pain unresponsive to medical management, evaluation for entrapment of the pudendal nerve may be pursued.470 The nerve may be tested for delays in conduction; if abnormality is present, successful relief of pain by pudendal block (providing the diagnosis) may lead to surgery to release entrapment. There are four described approaches to surgical decompression of the pudendal nerve. All surgical methods involve neurolysis to eliminate the possible source of compression. The trans-gluteal approach is currently the most common and successful approach for pudendal neurolysis.471 This procedure was originally described by Professor Roger Robert from Centre Hospitalier Universitaire in Nantes, France. In a sequential, randomized, controlled trial, 71.4% of the surgery group compared with 13.3% of the non-surgery group had improved at 12 months.472
Good evidence about efficacy of pudendal nerve blocks in reducing pain, especially over the long term, is lacking because of use of multiple methods, medications (steroids and anesthetic agents), and time frames, as well as lack of randomized controlled trials which would be theoretically impossible to do in these cases.473
Nerve blocks are also discussed in Annotation L, The Pelvic Floor (Link to Annotation L) Also see https://www.pudendalhope.info/node/11 for information on nerve blocks.
Pulsed radiofrequency (PRF) for pudendal neuralgia
PRF is a technique increasingly used by specialists to alleviate several types of pain by directing an electrical field current and heat bursts through a catheter needle tip to targeted nerves or tissues without causing damage.474 It can be considered for use for PN if conservative treatments, including PNB, have been tried without helping, prior to a decision for surgery. Although the mode of action is not well understood, the treatment seems to “reset” the nerves and, in many cases, diminish pain. PRF, using relatively high voltage applied near a neural tissue in short pulses, causes a “lesion” that will stop nociceptive (A-delta and C fibers) input into the central nervous system without having a destructive effect on motor or sensory fibers.475 476 Pulsed radio frequency (PRF) may interfere with normal cell function at the dorsal root ganglion that is induced through changes in myelin and changes in the intracellular axonal components of the pain afferents.477
A prospective, randomized controlled trial comparing the use of pudendal nerve block (PNB) alone or PNB followed by PRF treatment in patients with pudendal neuralgia showed that while both groups had improvement in pain scores, pain scores were lower, including at 3 month follow up, in patients who received the PRF treatment.478
In a 2021 case series of 20 patients with PN resistant to conservative treatment, PRF was employed via a 22 gauge needle with 5 mm active tip RF, placed transvaginally 1.5 cm medial to the ischial spine. A 45 V radiofrequency current was delivered in pulses of 20 ms with a frequency of 2 Hz. Treatment duration was 240 seconds. In case of bilateral pain, the procedure was performed on both sides.479 Individual pain relief lasted between 6 weeks to six months. (One patient said she was “much worse” at three months and did not continue with this treatment). 79% of patients felt that they were “very much better” at three month follow up. The procedure was repeated as necessary since there were no adverse effects. 89% said they were “very much better” at six months’ follow up. The authors declared the following: Compared with other treatment options, both PNBs with local anesthetics and surgical decompression are less effective than PRF, with success rates of 31% to 35.9% for pudendal NBs and 60% for surgical decompression.480
Transcutaneous Electrical Nerve Stimulation (TENS) and vaginal diazepam
Transcutaneous electrical nerve stimulation (TENS) is a well known nonpharmacologic, noninvasive treatment to relieve pain from multiple etiologies. TENS reduces pain through both peripheral and central mechanisms. Centrally, sites in the spinal cord and brainstem that utilize opioid, serotonin, and muscarinic receptors are activated by TENS. In peripheral sites, TENS application produces analgesia though opioid and alpha-2 noradrenergic receptors.481 It is thought that pain relief is achieved either through the gate-control theory, which postulates presynaptic inhibition of pain signals by stimulation of non-nociceptive afferent neurons and/or a mechanism that uses the descending pain suppression pathway originating in the cortex, which is called supraspinal inhibition.482
Weekly intravaginal TENS sessions combined with pelvic floor rehabilitation and biofeedback brought improvement in vulvar pain in 75.8% of all cases.483 In a double-arm randomized placebo-controlled trial, a vaginal probe delivered either sham (placebo) or functional TENS in a clinical setting twice a week for three weeks. There was improvement in all main outcome measures in 40 women with vestibulodynia.484 A longitudinal prospective cohort study was performed on 39 women, ages 19-41, with therapy-resistant LPV/PVD. 65% had secondary LPV/PVD. The patients received TENS treatment at home after training with a pelvic floor physical therapist. In this study, TENS electrodes were applied to the shaved labia majora in a V shape bilaterally two to three times a day for 6-8 weeks. Patients stopped the treatment if they improved to their own satisfaction.485 The efficacy of this therapeutic modality was noted immediately and, after a follow-up of 10 months, only 4% of the patients needed vestibulectomy compared with 23% in a previous study. Distress related to the psychosexual effects of the LPV/PVD, was significantly reduced and many were able to resume sexual relations. When noting the limitations of the study, the authors pointed to possibly confounding variables including the patients’ sense of being in control of the treatment and emotionally supported by the physical therapist, as well as becoming more comfortable touching their own bodies.
TENS alone versus TENS with intravaginal diazepam
Diazepam is a benzodiazepine medication mostly used for anxiety, spasticity associated with upper motor neuron disorders, adjunctive therapy for muscle spasms, preoperative anxiety relief, management of certain refractory epilepsy patients, as adjunct in severe recurrent convulsive seizures, and as an adjunct in status epilepticus.486 It has been used off-label by vulvovaginal specialists who have theorized that it might help if applied vaginally in cases of vulvodynia where there is high tone pelvic floor activity. A 2019 study examined the pharmacokinetic and adverse event profile of a single 10 mg vaginal suppository in healthy women. Serum samples were done at 0, 45, 90, 120, and 180 minutes; 8, 24, and 72 hours; and 1 week following administration of the suppository in 8 women. A mean peak diazepam concentration (Cmax) of 31.0 ng/mL was detected at a mean time (Tmax) of 3.1 hours after suppository placement. The bioavailability was found to be 70.5%, and the mean terminal elimination half-life was 82 hours. Three patients reported fatigue. Conclusions were that vaginal administration of diazepam results in lower peak serum plasma concentration, longer time to peak concentration, and lower bioavailability than standard oral use, but that with diazepam’s long half-life, accumulating levels would occur with chronic daily doses, and steady-state levels would not be reached for up to 1 week, suggesting a better safety profile with intermittent use.487
A 2010 retrospective chart review of 26 patients who received vaginal diazepam in addition to pelvic floor physical therapy and trigger point injections showed that 25 reported amelioration of symptoms.488
Most studies have not shown significant efficacy. In one, 21 subjects with high tone pelvic floor muscles in the setting of vulvodynia, used 10 mg of vaginal diazepam nightly for four weeks without improvement in resting EMG levels or subjective outcomes compared with placebo.489 In another randomized placebo controlled trial, 49 women were randomized to using either vaginal diazepam suppositories or placebo. There were no differences in outcomes for the two groups. 490
A 2020 systematic review of available studies491 concluded that only the following study (Murina, et al), showed statistical improvement in the diazepam group. In this double-arm randomized placebo controlled trial, researchers looked at the efficacy of intravaginal diazepam plus intravaginal TENS versus placebo and intravaginal TENS for the treatment of vestibulodynia with high tone pelvic floor dysfunction in a study involving 65 women.492
Both groups reported improved pain values, sexual functioning, and objective parameters, such as pelvic floor muscle tone and current perception threshold (CPT) values of vestibular nerve fibers compared to before treatment. However, very few statistically significant differences in outcomes between the two groups were observed. This study confirmed the value of TENS use alone. In addition, though, it was observed that measurements of pelvic floor EMG values dropped significantly in the group using vaginal diazepam. In other words, there was less hyperactivity of the muscles. Patients were better able to relax the pelvic floor muscles after contraction in this group. Patients also reported a significant reduction of Marinoff dyspareunia scores in the diazepam group that occurred over the 60 days of therapy. Another observation was that CPT testing, which quantifies the functional integrity of specific afferent nerve fibers from the periphery to the central nervous system, showed a reduction by half compared with the placebo group and the difference from baseline values was statistically significant. The authors theorized that diazepam may act indirectly on the pain as decreased muscle spasms are pivotal for the neuropathic reflexes occurring in hyperalgesia. They concluded that the combination of TENS and diazepam might, indeed, be helpful for women with high tone vulvodynia.493
Neurotoxic agent for muscle relaxing effect-Botulinum type A
Botulinum type A (Botox) inhibits release, at the neuromuscular junction, of pain-producing glutamate and substance-P from nociceptive neurons; inhibiting these nociceptors can decrease peripheral and central sensitization and pelvic floor hypertonicity associated with vulvodynia.494 It has also been suggested that the antinociceptive mechanism of botulinum toxins (BTXs) applied to the nerve endings not only affects the primary afferent neurons but also acts on the dorsal root ganglion, and spinal dorsal horn through retrograde axonal transport.495
By 2004, several studies had evaluated Botox, but it had not yet proven to be effective for vulvodynia. In one randomized controlled trial in women with LPV/PVD, a low dose (20 U) injection into the bulbocavernosus muscles brought the same relief as placebo.496 However, an uncontrolled study reported favorable outcomes with injections up to 100 U.497 A small randomized controlled trial using 50 U and 100 U indicated no difference in vestibular pain sensitivity at 3 months after treatment between women who received Botox or those who received placebo. The authors indicated, however, that repeated high-dose injections could reduce pain over six months.498
Four of seven serotypes of Clostridium botulinum toxin are currently FDA-approved in the USA for diverse indications. They differ in their reaction mechanisms, durations of effect, and side effects; different doses are required for each type of toxin.
Serotype A toxins are:
- OnabotulinumtoxinA (onaBoNTA),
- AbobotulinumtoxinA (aboBoNTA)
- incobotulinumtoxinA (incoBonNTA)
- RimabotulinumtoxinB (rimaBoNTB) (Serotype B toxin)
In 1997, OnaBoNTA, the first with FDA approval, was being used for treatment of painful, non- bladder pelvic pain in; successful treatment of one woman with vaginismus was reported.499 By 2004, a series of uncontrolled, open-label studies examined onaBoNTA use for the treatment of levator ani spasm, showing a reduction in resting pelvic floor pressure and significant improvement in dyspareunia and non-menstrual pelvic pain.500 In 2006, a subsequent masked, randomized, placebo-controlled trial in 60 women with chronic pelvic pain (CPP) revealed improvement in pain in both active drug and placebo groups, with improvement in non-menstrual pelvic pain only found in the active drug group.501 Except for case reports, case series and uncontrolled trials make up the large field of literature on the use of onaBoNTa to treat vaginismus, vulvodynia, and CPP. Karp et al retrieved 38 database reports with analyzable information. The most frequent toxin used was onaBoNTA 10-300 units. This database information is impressively positive. 58-100% of patients with CPP and 71-100% of women with vulvovaginal provoked pain improved. Serious adverse events occurred with more frequent higher doses. The researchers concluded that onaBoNTA can be safely and tolerably injected into pelvic floor muscles in women as an out-patient procedure. They also indicate the crucial need for high quality research on botulinum toxicity. 502
Therapeutic immune system intervention, a promising treatment
Research has shown that there exists in all women, pain-free or not, an enhanced innate immune system localized to the vulvar vestibule and controlled partially by regional fibroblasts. Toll-like receptors (TLR) are a class of innate immune receptors that rapidly respond to microbial assaults, always possible in the vulva and vagina. They identify molecular patterns from the assaults. Human vulvar fibroblasts express a wide spectrum of toll -like receptors that trigger production of inflammatory mediators associated with chronic pain. A significantly higher TLr-mediated proinflammatory response was observed in fibroblasts from LPV cases.503 A 2018 study by Falsetta et al found a significantly greater Toll-like receptor-mediated proinflammatory reaction in vestibular fibroblasts from women with LPV/PVD versus pain-free controls. This discovery advanced thinking that an upregulated immune reaction system might be part of the pathophysiology of vulvodynia and could be targeted therapeutically. Specialized pro-resolving mediators (SPMs), lipids produced endogenously within the body, hold promise as an LPV treatment by resolving inflammation without impairing host defense. Ten of 13 commercially available SPMs reduced IL-6 and PGE2 production by vulvar fibroblasts, administered either before or after inflammatory stimulation.504 505 At the same time, animal models have indicated that the neuronal hypersensitivity in LPV/PVD may be achieved by nociceptor axon proliferation associated with inflammatory cell-derived angiotensin II AT2 action on AT2 receptors. This action could be blocked in an animal model of LPV/PVD and could also be a potential future therapeutic possibility.506 507
Using a mouse model, created de novo, Falsetta et al tested the effect of maresin I and the DHA on vulvar sensitivity, and found that both were effective in raising sensitivity thresholds reflective of increased tolerance of force and presumably reduced sensitivity. thresholds. Using a murine pain model, coupling proinflammatory mediator quantification with mechanical sensitivity threshold determination, topical treatment with the SPM, maresin I, was also effective in reducing PGE2 in vulvar fibroblasts and rapidly restored mouse sensitivity thresholds. On the whole, SPMs amd even the fatty acids from which they are derived, are effective in reducing pro-nociceptive IL-6 and PGE2 levels and restoring pain thresholds to baseline levels in mice.508
With the conviction that the proposal and observations are valid, the phenomenon of regional enhanced responsiveness to innate immune mediators may precede LPV/PVD onset and bring about early stage LPV/PVD pathogenesis. Therapeutic intervention at this early stage might bring hope of effective new options and ideally primary prevention.509
Alternative nonmedical treatments
There are scant data supporting the efficacy of alternative treatment such as hypnosis or acupuncture which are used by women with vulvodynia. A small pilot study published in 2010 showed that after ten one hour sessions with acupuncture needles placed by specialists in Chinese traditional medicine, most women claimed some improvement in symptoms, but results were only statistically significant for improvement in manual genital stimulation and feelings of helplessness.510
In 2015, a randomized wait-list controlled pilot study examining the use of acupuncture in women with vulvodynia was published. Thirty-six women with vulvodynia were randomly assigned to the acupuncture (twice weekly treatment for five weeks) or the wait-list control group. Results indicated that, after treatment, women who received acupuncture reported significantly less vulvar pain and dyspareunia with improvements in overall sexual function compared with the control group. Feelings of sexual desire, arousal, lubrication, ability to orgasm or sexual satisfaction were not improved in women with acupuncture treatment. Because the treatment produced no adverse and some positive effects, more extensive RCT studies are warranted.511
References
- Bohm-Starke N, Ramsay KW, Lytsy P, Nordgren B, Sjöberg I, Moberg K, Flink I. Treatment of Provoked Vulvodynia: A Systematic Review. J Sex Med. 2022 May;19(5):789-808. doi: 10.1016/j.jsxm.2022.02.008. Epub 2022 Mar 21.PMID:35331660
- https://www.sbu.se/en/ongoing-projects/development-of-a-core-outcome-set-for-treatment-of-provoked-vestibulodynia/
- Falsetta M, et al. Specialized Pro-resolving Mediators Reduce Pro-nociceptive Inflammatory Mediator Production in Models of Localized Provoked Vulvodynia. J Pain. 2021 Oct;22(10):1195-1209. doi: 10.1016/j.jpain.2021.03.144. Epub 2021 Apr 1.
- Harlow BL et al. The Association Between Immune-Related Conditions Across the Life-Course and Provoked Vulvodynia. Pain. 2023 Aug;24(8): 1415-1422.
- Farmer MA. Pathophysiology of pain: Peripheral and Central. In: Female Sexual Pain Disorders, Second Edition, 2021. Goldstein AT, Pukall CF, Goldstein I, Ed. Oxford, England. John Wiley & Sons. p15-30.
- Farmer MA. Pathophysiology of pain: Peripheral and Central. In: Female Sexual Pain Disorders, Evaluation and Management. Second Edition, 2021. Goldstein AT, Pukall CF, Goldstein I, Ed. Oxford, England. John Wiley & Sons. 15-30.
- Hladnik A, et al. Functional neuroanatomy of nociception and pain. Periodicum Biologorum. 2015; 117(2):195-204.
- Farmer M. Pathophysiology of pain: peripheral and central. In Female Sexual Pain Disorders, Evaluation and Management; second edition. 2021. Goldstein AT, Pukall CF, Goldstein I, Ed. Oxford, England. John Wiley & Sons.
- Latremoliere A, Woolff C. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J Pain.2009 Sep;10(9):895-926.
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014. 14:217–31.
- Vanderwall AG, Milligan ED. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Frontiers in Immunology. 2019; 10:3009.
- Latremoliere A, Woolff C. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J Pain. 2009 Sep;10(9):895-926.
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688.
- Gold MS, Gerhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010; 16:1248.
- Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014;37:343-355.
- Ji RR, Naackley A, Huh Y, et al. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018; 129: 343-66.
- Ji RR, Naackley A, Huh Y, et al. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018; 129: 343-66.
- Campbell JN, Meyer RA. Mechanisms of Neuropathic pain. Neuron.2006;52(1):77-92
- Latremoliere A, Woolff C. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J Pain; 2009 Sep;10(9):895-926.
- Scerbo T, Colasurdo J., et al. Measurement Properties of the Central Sensitization Inventory: A Systematic Review. Pain Pract. 2018; Apr;18(4):544-554.
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009 Sep;10(9):895-926. doi: 10.1016/j.jpain.2009.06.012. PMID: 19712899; PMCID: PMC2750819.
- Ossipov M, Dussor G, Porreca F. Central modulation of pain. J Clin. Invest. 2010 120(11):3779-3787.
- Bouorne S, et al. Basic Anatomy and Physiology of Pain Pathways. Neurosurg Clin North America. 2014;25(4): 629-638
- JI RR, Nackley A, Huh Y et al. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018; 129:343-66.
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009 Sep;10(9):895-926. doi: 10.1016/j.j J Pain.2009.06.012. PMID: 19712899; PMCID: PMC2750819.
- Roberts WJ. A hypothesis in the physiological basis for causalgia and related pain. Pain 1986; 24:297-31
- Torres-Cueco R, Nohales-Alfonso F. Vulvodynia- It Is Time to Accept a New Understanding from a Neurobiological Perspective. Int J Environ Res Public Health. 2021. Jun; 18 (12): 6639.
- Hladnik A, et al. Functional neuroanatomy of nociception and pain. Periodicum Biologorum. 2015; 117(2):195-204.
- Woolf CJ. What is this thing called pain? J Clin Invest. Nov 1, 2010; 120(11): 3742-44.
- Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the Third World Congress of World Institute of Pain, Barcelona 2004. Pain Practice.2005.5(2):85-94.
- Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 2021 May 29;397(10289):2098-2110. doi: 10.1016/S0140-6736(21)00392-5. PMID: 34062144.
- Meade E, Garvey M. The role of Neuro-Immune Interaction in Chronic Pain conditions: Functional Somatic Syndrome: Neurogenic Inflammation and Peripheral Neuropathy. Int J Mol Science. 2022 Aug; 23(15):8574.
- Woolf CJ. 2010 What is this thing called pain? J Clin Invest. Nov 1; 120(11): 3742-44.
- Micheletti L, Radici G, Lynch PJ. Provoked Vestibulodynia: inflammatory, neuropathic or dysfunctional Pain? A neurobiological perspective. J Obstet Gynecol.2014; 34(4):255-258.
- Meade E, Garvey M. The role of Neuro-Immune Interaction in Chronic Pain conditions: Functional Somatic Syndrome: Neurogenic Inflammation and Peripheral Neuropathy. Int J Mol Science. 2022 Aug; 23(15):8574.
- Henzell H, Berzins K, Langford JP. Provoked vestibulodynia: current perspectives. Int J Women’s Health. 2017; 9:631-642.
- https://en.wikipedia.org/wiki/DSM-5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. Washington, DC: APA; 2013
- Vieira-Baptista, Pedro MD; Lima-Silva, Joana MD. Is the DSM-V Leading to the Non Diagnosis of Vulvodynia?. Journal of Lower Genital Tract Disease 20(4):p 354-355, October 2016. DOI:10.1097/LGT.0000000000000250
- Henzell H, Berzins K, Langford JP. Provoked vestibulodynia: current perspectives. Int J Womens Health. 2017 Sep 11;9:631-642. doi: 10.2147/IJWH.S113416. PMID: 28979166; PMCID: PMC5602415.
- Vieira-Baptista, Pedro MD; Lima-Silva, Joana MD. Is the DSM-V Leading to the Non Diagnosis of Vulvodynia?. Journal of Lower Genital Tract Disease 20(4):p 354-355, October 2016. | DOI: 10.1097/LGT.0000000000000250
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Torres-Cueco R, Nohales-Alfonso F. Vulvodynia-It Is Time to Accept a New Understanding from a Neurobiological Perspective. Int J Environ Res Public Health. 2021 Jun 21;18(12):6639. doi: 10.3390/ijerph18126639. PMID: 34205495; PMCID: PMC8296499.
- Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, Coady D; consensus vulvar pain terminology committee of the International Society for the Study of Vulvovaginal Disease (ISSVD), the International Society for the Study of Womenʼs Sexual Health (ISSWSH), and the International Pelvic Pain Society (IPPS). 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. J Low Genit Tract Dis. 2016 Apr;20(2):126-30. doi: 10.1097/LGT.0000000000000190. PMID: 27002677
- Adapted from the 2015 Consensus Statement which applied these descriptors to vulvodynia alone but which can also be applied to any pain.
- Bornstein, Jacob MD, MPA1; Preti, Mario MD2; Simon, James A. MD3, et al. Descriptors of Vulvodynia: A Multi Societal Definition Consensus (International Society for the Study of Vulvovaginal Disease, the International Society for the Study of Women Sexual Health, and the International Pelvic Pain Society). Journal of Lower Genital Tract Disease.April 2019.23(2):p 161-163, | DOI: 10.1097/LGT.0000000000000461
- Bornstein J, Maman M, Abramovici H. “Primary” versus “secondary” vulvar vestibulitis: One disease, two variants. Am J Obstet Gynecol. 2001;184:28–31.
- Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstet Gynecol. 2011 Jun;117(6):1307-1313. doi: 10.1097/AOG.0b013e31821c33dc. PMID: 21606740; PMCID: PMC3104470.
- Adapted from the 2015 Consensus Statement; the original was in alphabetical order.
- Merlino L, Titi L, Pugliese F, D’Ovidio G, Senatori R, Rocca CD, Piccioni MG. Vulvodynia: Pain Management Strategies. Pharmaceuticals (Basel). 2022 Dec 5;15(12):1514. doi: 10.3390/ph15121514. PMID: 36558965; PMCID: PMC9781267.
- Goldstein AT. Diagnostic and treatment algorithm for women with vulvodynia and sexual pain disorders. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and Sons, 2021. p157-162.
- Sadownik LA. Etiology, diagnosis, and clinical management of vulvodynia. Int J Womens Health. 2014;6:437–49.
- Clare CA, Yeh J. Vulvodynia in adolescence: childhood vulvar pain syndromes. J Pediatr Adolesc Gynecol. 2011;24(3):110–115.
- Harlow, B. L. et al. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population based estimates from 2 geographical regions. 2014. Am. J. Obstet. Gynecol. 210, 40.e1–8. – DOI
- Reed BD, Harlow SD, Sen A, et al. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am J Obstet Gynecol. 2012;206(2):170.e1–e9.
- Mitro SD, Harlow SD, Randolph JF, Reed BD. Chronic vulvar pain in a cohort of post-menopausal women: Atrophy or Vulvodynia? Womens Midlife Health. 2016;2:4. doi: 10.1186/s40695-016-0017-z. Epub 2016 Jun 9. PMID: 28127441; PMCID: PMC5260822.
- Al-Safi ZA, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertility and sterility. 2014 Apr;101(4):905–15.
- Edwards L, Subsets of vulvodynia: overlapping characteristics. J Reprod Med 2004; 49(11):883-887
- Reed BD, Gorenflo DW, Haefner HK. Generalized vulvar dysesthesia vs vestibulodynia. Are they distinct diagnoses? J Reprod Med 2003; 48(11): 858-864.
- Dellon AL, Coady D, Harris, D. Pelvic Pain of Pudendal Nerve origin: Surgical Outcomes and Learning Curve Lessons. J Reconstructive Microsurg. 2015;31: 283-290.
- Klifto KM, Dellon AL. Persistent Genital Arousal Disorder: Review of Pertinent Peripheral Nerves. Sex Med Rev. April 2020;8(2): 265-273.
- Reed BD, Harlow SD, Piegue MA, Sen A. Remission, relapse and persistence of vulvodynia: a longitudinal population-based study. J Womens Health (Larchmt). 2016;25(3): 276-283.
- Xie Y., Shi L., Wu E., Veasley C., Dade C. Economic burden and quality of life of vulvodynia in the United States. Curr Med Res Opin, 2012. 28:601-08.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital tract discomfort. Am. J.Obstet .Gynecol. 2001;185:545-550.
- Harlow BL, Stewart EG. A population based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J. Am. Med. Women’s Assoc, 2003.58:82-88.
- Harlow BL, Kunitz CG, Nguyen RHN, et al. 2014. Prevalence of symptoms consistent with a diagnosis of vulvodynia:population based estimates from 2 geographic regions. Am J Obstet Gynecol. 210, 40.e1-8.
- Arnold LD, Bachmann GA, Rosen R, Rhoads GG. Assessment of vulvodynia symptoms in a sample of US women: a prevalence survey with a nested case control study. Am J Obstet Gynecol. 2007 Feb;196(2):128.e1-6. doi: 10.1016/j.ajog.2006.07.047. PMID: 17306651; PMCID: PMC2746064.
- Gomez I, Coronado PJ, Martin CM, Alonso R & Guisasola-Campa FJ. Study on prevalence ad factors associated to vulvodynia in Spain. Eur. J. Obstet.Gynecol.Reprod.Biol, 2019. 240.121-124.
- Viera-Baptista P, Lima-Silva J, Cavaco-Gomes J, Bieres J. 2014. Prevalence of vulvodynia and risk factors for the condition in Portugal. Int J Gynaecol. Obstet. 127: 283-287.
- Pathak D, Agrawal S, & Dhali TK. Prevalence of and risk factors for vulvar diseases in Nepal: a hospital-based study. Int.J.Dermatol 2011. 50: 161-167.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nature Reviews Disease Primers. 2020. 6:36.
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States, prevalence and predictors. JAMA.1999;281, 537-544.
- Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch. Sex. Behav, 2011. 40, 877-899.
- Mitchell KR, et al. Sexual function: findings from the third national survey of sexual attitudes and lifestyles (Natsal-3). Lancet 2013. 382:1817-1829.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Nature Reviews/Disease Primers 2020. 6:36.
- Lev-Sagie A, Witkin SS. Recent advances in understanding provoked vestibulodynia. F1000Res. 2016 Oct 26;5:2581. doi: 10.12688/f1000research.9603.1. PMID: 27853523; PMCID: PMC5089138.
- Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. J Pain. 2013 Jun;14(6):579-89. doi: 10.1016/j.jpain.2013.01.767. Epub 2013 Apr 9. PMID: 23578957; PMCID: PMC3672331
- Bhatt RR, Gupta A, Rapkin A, Kilpatrick LA, Hamadani K, Pazmany E, Van Oudenhove L, Stains J, Aerts L, Enzlin P, Tillisch K, Mayer EA, Labus JS. Altered gray matter volume in sensorimotor and thalamic regions associated with pain in localized provoked vulvodynia: a voxel-based morphometry study. Pain. 2019 Jul;160(7):1529-1540. doi: 10.1097/j.pain.0000000000001532. PMID: 30817440; PMCID: PMC6586504.
- Gupta A, Woodworth DC, Ellingson BM, Rapkin AJ, Naliboff B, Kilpatrick LA, Stains J, Masghati S, Tillisch K, Mayer EA, Labus JS. Disease-Related Microstructural Differences in the Brain in Women With Provoked Vestibulodynia. J Pain. 2018 May;19(5):528.e1-528.e15. doi: 10.1016/j.jpain.2017.12.269. Epub 2018 Jan 31. PMID: 29391213; PMCID: PMC5927835.
- Bogliatto, Fabrizio MD, PhD; Miletta, Michela MW. Vulvodynia: When a Paradigm Challenges the Management. Journal of Lower Genital Tract Disease. January, 2017. 21(1):p 85-86.| DOI: 10.1097/LGT.0000000000000263
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p333-347.
- Wesselmann U, Bonham B, Foster DC. Vulvodynia: Current state of the biological science. Pain. 2014, May; 155(9):1696-1701.
- Reed BD, McKee KS, Plegue MA, Park SK, Haefner HK, Harlow SD. Environmental exposure history and vulvodynia risk: a population based study. J Women’s Health. 2019;28:69-76
- Wesselmann U, Bonham B, Foster DC. Vulvodynia: Current state of the biological science. Pain. 2014, May, 155(9):1696-1701.
- Reed BD, McKee KS, Plegue MA, Park SK, Haefner HK, Harlow SD. Environmental exposure history and vulvodynia risk: a population based study. J. Women’s Health. 2019;28:69-76.
- Guo H, Callaway JB, Ting Jenny P-Y. Inflammasomes: Mechanism of Action, Role in Disease and Therapeutics. Nat Med. 2015 Jul; 2(7): 677-687.
- Bohm-Starke N, Hilliges M, Blomgren B, et al.: Increased blood flow and erythema in the posterior vestibular mucosa in vulvar vestibulitis. Obstet Gynecol. 2001; 98(6): 1067–74.
- Farage M, Singh M, Ledger WJ. Investigation of the sensitivity of a cross-polarized visualization system to detect subclinical erythema and dryness in women with vulvovaginitis. Am. J. Obstet Gynecol. 2009;201:20e1.
- Akopians AL, Rapkin AJ. Vulvodynia: The Role of Inflammation in the Etiology of Localized Provoked Pain of the Vulvar Vestibule (Vestibulodynia). Semin Reprod Med. 2015 Jul;33(4):239-45. doi: 10.1055/s-0035-1554919. Epub 2015 Jul 1. PMID: 26132928.
- Foster, DC. The Role of Inflammation in Vulvodynia. In: Female Sexual Pain Disorders. 2nd ed. Goldstein AT, Pukall C, Goldstein I, Editors. John Wiley & Sons. 2021. 31-41.
- Witkin SS, Linhares LM, Giraldo P. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynecol. 2007; 21(3): 347-354.
- Farmer MA. Pathophysiology of Pain: Peripheral and Central. In: Female Sexual Pain Disorders. 2nd edition. Goldstein AT, Pukall CF,Goldstein I, ed. John Wiley & Sons. 2021. p21
- Foster, DC. The Role of Inflammation in Vulvodynia. Figure 4.1: A proposed natural history of vulvodynia development. P. 36. In: Female Sexual Pain Disorders. 2nd ed. Goldstein AT, Pukall C, Goldstein I, Editors. John Wiley & Sons. 2021. 31-41
- Friedrich EG Jr: Vulvar vestibulitis syndrome. J Reprod Med. 1987; 32(2): 110–4.
- Pyka RE, Wilkinson EJ, Friedrich EG Jr, et al.: The histopathology of vulvar vestibulitis syndrome. Int J Gynecol Pathol. 1988; 7(3): 249–57
- Chaim W., Meriwether C., Gonik B., et al. Vulvar vestibulitis subjects undergoing surgical intervention: a descriptive analysis and histopathological correlates. Eur. J. Obstet. Gynecol Reprod. Biol. 1996; 68:165-168. Another study revealed subepithelial inflammatorycells, sometimes aggregated into lymph follicles and/or small groups of lymphocytes in vulvodynia cases and also in pain free controls. Staining necessary to detect mast cells was not done. The researchers concluded that the occurrence ofinflammatorycells in vestibular tissue is a normal finding and cannot serve as ahistologicalindicator ofvulvar vestibulitis.98Lundquist E.N., Hofer, P.A., Olofsson, JJ, and Sjoberg,I. Is vulvar vestibulitis an inflammatory condition? A comparison of histological findings in affected and healthy women. Acta Derm Venerol. 1997;77:319-322.
- Chadha S, Gianotten WL, Drogendijk AC, et al.: Histopathologic features of vulvar vestibulitis. Int J Gynecol Pathol. 1998; 17(1): 7–11.
- Bornstein J, Goldschmidt N, Sabo E. Hyperinnervation and mast cell activation may be used as histopathologic and diagnostic criteria for vulvar vestibulitis. Gynecol. Obstet. Invest.2004;58(3):171-178.
- Bornstein J, Cohen Y, Zarfati D. et al. Involvement of heparinase in the pathogenesis of localized vulvodynia. Int J Gynecol Pathol. 2008;27(1):136141.
- Halperin R, ZehaviS, Vaknin , et al. The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gynecol Obstet Invest. 2005; 59(2):75-79.
- Eva LJ, Rolfe KJ, MacLean AB, et al. Is localized, provoked vulvodynia an inflammatory condition? J. Reprod. Med. 2007; 52:379-84.
- Tommola P, Butzow R, Unkila-Kallio L et al. Activation of vestibule-associated lymphoid tissue in localized provoked vulvodynia. Am J Obstet Gynecol 2015; 212:476.e1-476.e8.
- Tommola P, Unkila-Kallio L, Paetau A, Meri S, Kaiso E, Pavonnen J. Immune activation enhances epithelial nerve growth in provoked vestibulodynia. Am. J. Obstet. Gynecol. 2016; 215:768.e1-8.
- Barry CM, Matusica D, Haberberger RV. Emerging Evidence of Macrophage Contribution to Hyperinnervation and Nociceptor Sensitization in Vulvodynia. Front Mol Neurosci 2019; 12:186.
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005. 23, 344–346. 10.1016/j.immuni.2005.10.001
- Farmer M. Pathophysiology of Pain: Peripheral and Central. In: Female Sexual Pain Disorders, 2nd edition. Goldstein AT, Pukall, CF, Goldstein I., Eds., Oxford, England. Wiley Blackwell & Sons. 2021: 15-30
- Wesselmann U., Bonham A., Foster D: Vulvodynia: Current state of the biological science. Pain. 2014;155(9)
- Bohm-Starke N, Hilliges M, Falconer C, et al.: Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1998; 46(4): 256–60.
- Foster DC, Hasday JD. Elevated tissue levels of interleukin-lp and tumor necrosis factor-alpha in vulvar vestibulitis. Obstet Gynecol 1997; 89(2):291-196.
- Foster DC, Piekarz KH, Murant TL, et al. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am. J. Obstet. Gynecol. 2007; 196(4): 346.e1-8.
- Falsetta M, Foster DC, Woeller CF, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015; 123(1):38e1-12.
- Harlow BL, Wei He, and Nguyen R. Allergic reactions and risk of vulvodynia. Ann. Epidemiol. 2009 Nov;19(11):771
- Bornstein J., Cohen Y., Zarfati D., et al. Involvement of heparinase in the pathogenesis of localized vulvodynia. Int.J.Gynecol. Pathol. 2008; 27:136-141
- Goetsch M.F, vestibulodynia and controls: a prospective study. Am. J. Obstet. Gynecol. 2010; 202;614-E1-614.E8.
- Leclair, C.M., Goetsch, M.F., Korcheva, V.B. et al. Differences in primary compared with secondary vestibulodynia by immunochemistry. Obstet. Gynecol. 2011;117:1307-1313.
- Halperin R., Zehavi W., Vaknin Z., et al. The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gynecol. Obstet. Invest. 2005; 59:75-79
- Willis, SK, Aiello AE, Chatterjea D, Nelson JA, Hibberd PL, Harlow BL. Characterizing differences in thymic function in women with and without vulvodynia: a community-based study. J. Lower Genital Tract Disease. 2021;25(4): 296-302
- Green J, Christimas P, Goldmeier D, Byrne M, Kocsis A, editors. A review of physical and psychological factors in vulvar vestibulitis syndrome.Int J STD AIDS.2001;12:705–709.
- Gunter J. Vulvodynia: New thoughts on a devastating condition. Obstet Gynecol Surv.2007;62:812–819.
- Rogstad KE. Vulvar vestibulitis: aetiology, diagnosis and treatment. Int J STD AIDS.2000;11:557–562.
- Harlow BL, Caron RE, Parker SE, Chatterjea D, Fox MP, Nguyen RHN. Recurrent Yeast Infections and Vulvodynia: Can We Believe Associations Based on Self-Reported Data? J Womens Health (Larchmt). 2017 Oct;26(10):1069-1076. doi: 10.1089/jwh.2016.5777. Epub 2017 Jul 7. PMID: 28686502; PMC51936.
- Foster DC, Falsetta ML, Woeller CF, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia- afflicted and pain-free women. Pain. 2015;156(3):386-96
- Foster DC, Falsetta ML, Woeller CF, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia-afflicted and pain-free women. Pain. 2015;156(3):386-96.
- Falsetta M, Foster DC, Woeller CF, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015; 123(1):38e1-12.
- Ji RR. Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. NAT Rev.Drug Discov. 2014; 13:533-548
- Farmer MA. What is special about the vulvar vestibule? Pain. 2015 Mar;156(3):359-360. doi: 10.1097/j.pain.0000000000000094. PMID: 25687538; PMCID: PMC4335348.
- Falsetta M, Foster DC, Woeller CF, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015; 123(1):38e1-12.
- Farmer M. What is special about the vulvar vestibule. Pain. 2015 Mra;156(3):351-360.
- Lev-Sagie A, Witkin SS. Recent advances in understanding provoked vestibulodynia. F1000Res. 2016 Oct 26;5:2581. doi: 10.12688/f1000research.9603.1. PMID: 27853523; PMCID: PMC5089138.
- Foster DC, Falsetta ML, Woeller CF, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia- afflicted and pain-free women. Pain. 2015;156(3):386-96.
- Basaran M, et al. Hymen sparing surgery for imperforate hymen; case reports and review of the literature. J Pediatr Adolesc Gynecol. 2009;22(4):e61–e64.
- Selo-ojeme DO, Paranjothy S, Onwude JL. Interstitial cystitis co-existing with vulvar vestibulitis in a 4 year old girl. Int Urogynecol J. 2002;13:261-262.
- Farmer MA. What is special about the vulvar vestibule? Pain. 2015. 156:359-360.
- Torres-Cueco R, Nohales-Alfonzo F. Vulvodynia-It is Time to Accept a New Understanding from a Neurobiological Perspective. Int J Environ Res Public Health 2021 Jun 21 Jun: 18(12):6639.
- Torres-Cueco RT, Nohales-Alfonso F. 2021.Vulvodynia- It is Time to Accept New Understanding from a Neurobiological Perspective. Int J Environ Res Public Health. Jun: 18(12):6639-6665. doi:10.3390/ijerph18126639
- Wesselmann U, Bonham AD, Foster D. Vulvodynia: current state of the biologic science. Pain, 2016;155:1696-1701.
- Zorina-Lichtenwalter K, Melotos CB, et al. Genetic predictors of human chronic pain conditions. Neuroscience. 2016; 338; 3 December: 36-62.
- Gerber S, Witkin SS, Stucki D. Immunological and genetic characterization of women with vulvodynia. J.Med Life. 2008; Nov 15 1(4): 432
- Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol.2000;182:283–285.
- Gerber S, Bongiovanni AM, Ledger WJ, Witkin SS. Defective regulation of the pro-inflammatory immune response in women with vulvar vestibulitis syndrome.Am J Obstet Gynecol.2002;186:696–700
- Gerber S, Bongiovanni AM, Ledger WJ, Witkin SS. Interleukin-1B gene polymorphism in women with vulvar vestibular syndrome.Euro J Obstet Gynecol.2003;107:74–77
- Babula O, Danielsson L, Sjoberg I, Ledger WJ, Witkin SS. Altered distribution of mannose-binding lectin alleles at exon 1 codon 54 in women with vulvar vestibulitis syndrome. Am. J. Obstet. Gynecol 2014; 191:762-766. doi:10.1016/j.ajog.2004.03.073.
- Tommola P, Unkila-Kallio L, Paetau A, Meri S, Kaiso E, Pavonnen J. Immune activation enhances epithelial nerve growth in provoked vestibulodynia. Am. J. Obstet. Gynecol. 2016; 215:768.e1-8.
- Lev-Sagie A, Prus D, Linhares IM. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2009;200:303.e1.
- Babula O,Danielsson I, Sjoberg I, Ledger WJ, Witkin SS.. Altered distribution of mannose binding lectin alleles at exon 1 codon 54 in women with vulvar vestibulitis syndrome. Am. J. Obstet. Gynecol. 2004;191:762-766. doi:10.1016/j.ajog.2004.03.073.
- Babula O, Lazdane G, Kroica J, Ledger WJ, Witkin SS. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin Infect Dis. 2003;37:733-7
- Summerfield JA, Sumiya M, Levin M, Turner MW. 1997. Association of mutations in mannose binding protein gene with childhood infections in consecutive hospital series. BMJ;314:1229-32.
- Rogstad KE. Vulvar vestibulitis: aetiology, diagnosis and treatment. Int J STD AIDS, 2000;11:557-62.
- Chaban V. Estrogen and visceral nociception at the level of primary sensory neurons. Pain Res.Treatment. Vol. 2012, pp1-6
- Farmer M. Pathophysiology of Pain: Peripheral and Central. In: Female Sexual Pain Disorders, 2nd edition. Goldstein AT, Poukall CF, Goldstein I., Eds. Wiley Blackwell. 2021:15-30.
- Chaban V. Estrogen and visceral nociception at the level of primary sensory neurons. Pain Res.Treat. 2012; 960780.
- De Icco R, Cucinella L, De Paoli I, et al. Modulation of nociceptive threshold by combined hormonal contraceptives in women with oestrogen-withdrawal migraine attacks: a pilot study. J Headache Pain 2016. 17(1).
- De Icco R, Cucinella L, De Paoli I, et al. Modulation of nociceptive threshold by combined hormonal contraceptives in women with oestrogen-withdrawal migraine attacks: a pilot study. J Headache Pain 2016. 17(1):70
- Farmer M. Pathophysiology of Pain: Peripheral and Central. In: Female Sexual Pain Disorders, 2nd ed. Goldstein AT, Pukall CF, Goldstein I., eds. Wiley Blackwell, 2021.
- Hormonal contraceptives in women with oestrogen withdrawal migraine attacks: a pilot study. J Headache Pain. 2016;17(1):70.[pp1-6
- Sanoja R, Cervero J. Estrogen modulation of ovariectomy-induced hyperalgesia in adult mice. Eur.J.Pain. 2008;12(5):573-581.
- Meczekalski B, Podfigurna-Stopa A, Sznankiewicz A, et al. Functional hypothalamic amenorrhea: Current view on neuroendocrine aberrations. Gynecol Endocrinol. 2008; 24:4.
- Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil Steril 1987;4794
- Goldstein AT. Hormonal causes of dyspareunia. In: Female Sexual Pain Disorders, 2nd edition. Goldstein AT, Pukall CF, Goldstein I., Eds.Wiley Blackwell 2021.63-68
- Goldstein AT. Hormonal Causes of Dyspareunia. In: Female Sexual Pain Disorders, 2nd edition. Goldstein AT, Pukall CF, Goldstein I., Eds.Wiley Blackwell 2021.63-68
- Goldstein AT, Belkin Zr, Krapf JM, et al. Polymorphisms of the androgen receptor gene and hormonal contraceptive induced provoked vestibulodynia. J. Sex. Med. 2014;11:2764.
- Burrows LJ, Basha M, Goldstein AT. The effects of hormonal contraceptives on female sexuality: a review. J Sex Med. 2012;9:213.
- Johannesson U, de Broussard, Jansen GB, et al. Evidence of diffuse noxious inhibitory controls (DNIC)elicited by cold noxious stimulation in patients with provoked vestibulodynia. Pain. 2007;130:31
- Bohm-Starke N, Johannesson U, Hilliges M, et al. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med 2004;49:888.
- Battaglia C, Morotti E, Persico N, et al. Clitoral vascularization and sexual behavior in young patients treated with drospirenone-ethinyl estradiol or contraceptive vaginal ring: a prospective randomized pilot study. J. Sex. Med. 2014;11:471
- Bazin S, Bouchard C, Brisson J, et al. Vulvar vestibulitis syndrome: an exploratory case control study. Obstet.Gynecol. 1994;83:47
- Bouchard C, Brisson J, Fortier M, et al. Use of oral contraceptive pills and vulvar vestibulitis: a case control study. Am J. Epidemiol 2002;156:254.
- Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med 2008; 53;102.
- Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J. Sex. Med. 2007;4:1679.
- Burrows LJ, Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex. Med. 2013;1:30.
- Reed BD, Harlow SD, Legocki LJ, et al. Oral contraceptive use and risk of vulvodynia: a population based longitudinal study. BJOG. 2013;120(13):1678-1684.
- Kao A, Binik Y, et al. Challenging Atrophied Perspectives on Postmenopausal Dyspareunia: Systemic Description and Synthesis of Clinical Pain Characteristics. J Sex Marital. Ther. 2012; 38:128-150
- Mitro SD, Harlow SD, Randolph JF, Reed BD. Chronic vulvar pain in a cohort of postmenopausal women: Atrophy or vulvodynia? Women’s Midlife Health. 2016;2:4.
- Goetsch MF. Severe Postmenopausal Genital Pain Treated Successfully With Prolonged Estrogen Therapy: A Case Series and Narrative Review. J Low Genit Tract Dis. 2020 Oct;24(4):405-410. doi: 10.1097/LGT.0000000000000553. PMID: 32604213.
- Liao Z, Chakrabarty A, Mu Y, Bhattacherjee A, Goestch M, Leclair CM, Smith PG. A Local Inflammatory Renin-Angiotensin System Drives Sensory Axon Sprouting in Provoked Vestibulodynia. J Pain. 2017 May;18(5):511-525. doi: 10.1016/j.jpain.2016.12.008. Epub 2017 Jan 3. PMID: 28062309; PMCID: PMC6261484.
- Griebling TL, Liao Z, Smith PG. Systemic and topical hormone therapies reduce vaginal innervation density in postmenopausal women. Menopause 2012; 19:630-635.
- Davilla GW, Singh A, Kurapanagiotou I, et al. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003;382-388.
- Goetsch MF. Where does postmenopausal pain hurt? A cross-sectional report. Menopause. 2022 June 1; 29(6); 646-653
- Hilliges M, Falconer C, et al. Innervation of the human vaginal mucosa as revealed by PGP 9.5 immunohistochemistry. Acta Anat 1995; 153: 119-126.
- Liao Z, Chakrabarty A, Mu Y, et al. A local inflammatory renin- angiotensin system drives sensory axon sprouting in provoked vestibulodynia. J Pain. 2017; 18:511–52.
- Goetsch MF. Where does postmenopausal pain hurt? A cross-sectional report. Menopause. 2022 June 1; 29(6); 646-653
- Mateos-Aparicio P, Rodriguez-Moreno A. The Impact of Studying Brain Plasticity. Front Cell Neurosci. 2019;13:66.
- Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012 Sep;13(9):910-20. doi: 10.1016/j.jpain.2012.06.006. PMID: 22958875; PMCID: PMC3495612.
- Reissing ED, Brown C, Lord MJ, et al.: Pelvic floor muscle functioning in women with vulvar vestibulitis syndrome. J Psychosom Obstet Gynaecol. 2005;26(2):107–13. 10.1080/01443610400023106
- Foster DC, Falsetta ML, Woeller CF, et al. Site-specfic mesenchymal control of inflammatory pain to yeast challenge in vulvodynia- afflicted and pain-free women. Pain. 2015;156(3):386-96.
- Falsetta M, Foster DC, Woeller CF, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015; 123(1):38e1-12.
- Farmer MA. What is special about the vulvar vestibule? Pain. 2015 Mar;156(3):359-360. doi: 10.1097/j.pain.0000000000000094. PMID: 25687538; PMCID: PMC4335348.
- Lev-Sagie A, Witkin SS. Recent advances in understanding provoked vestibulodynia. F1000Res. 2016; 5: 2581.
- Bergeron S, Binik YM, .Khalife S, Pagidas K, Glazer HI, Meana M, Amsel R. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
- Farmer MA, Maykut CA, et al. Psychophysical properties of female genital sensation. Pain. November 2013;154(11):2277-2286.
- Farmer MA, Maykut CA, et al. Psychophysical properties of female genital sensation. Pain. November 2013;154(11):2277-2286.
- Farmer MA, Maykut CA, et al. Psychophysical properties of female genital sensation. Pain. November 2013;154(11):2277-2286.
- Farmer MA, Maykut CA, et al. Psychophysical properties of female genital sensation. Pain. November 2013;154(11):2277-2286
- Farmer MA, Maykut CA, et al. Psychophysical properties of female genital sensation. Pain. November 2013;154(11):2277-2286.
- Berkeley KJ, Hotts H, et al. Functional properties of afferent fibers supplying reproductive and other pelvic organs in pelvic nerve of the rat. J Neurophys. 1990;63:256-72.
- Farmer MA, Maykut CA, et al. Psychophysical properties of female genital sensation. Pain. November 2013;154(11):2277-2286.
- Barry CM, Hulgol KK, Haberberger RV. New models to study vulvodynia: Hyperinnervation and nociceptor sensitization in the female genital tract. Neurol Regeneration Research. 2018;13(12):2096-2097.
- Westrom LV, Willen R. Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1998; 46(4): 256-60.
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1998;46(4):256-60. doi: 10.1159/000010045. PMID: 9813445.
- Bohm-Starke N,203Hilliges M, Falconer C, et al. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest;1999;48:270.
- Halperin R, Zehavi S, Vaknin Z et al. The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gynecol Obstet Invest. 2005; 59(2):75-9
- Tommola P, Unkila-Kallio L. Paetau A, et al. Immune activation enhances epithelial nerve growth in provoked vestibulodynia. Am J Obstet Gynecol 2016;pii.S0002-9378(16)30472-0
- Tympanidis P, Casula MA, Yiangou Y, et al. Increased vanilloid receptor VT1 innervation in vulvodynia. Eur. J. Pain. 2004; 8:129-33.
- Tympanidis P, Casula MA, Yiangou Y, et al. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur. J. Pain.2004; 8:129-33.
- Leclair CM, Goetsch MF, Korcheva VB, et al. Differences in primary compared with secondary vestibulodynia by immunohistochemistry.Obstet Gynecol. 2011;117(6):1307-13.
- Liao A, Chakrabarty A, Mu Y, et al. A local inflammatory renin-angiotensin system drives sensory axon sprouting in provoked vestibulodynia. J Pain. 2017; 18:511-525.
- Velikonja L, Giovannetti O, et al. Innervation of the human vulvar vestibule: A comprehensive review. Clinical Anatomy. 2023; 36:18-27.
- Velikonja L, Giovannetti O, et al. Innervation of the human vulvar vestibule: A comprehensive review. Clinical Anatomy. 2023; 36:18-27.
- Foster, C. The Role of inflammation in Vulvodynia In: Female Sexual Pain Disorders, Second Edition, 2021. AT Goldstein, CF Pukall, Irwin Goldstein, ED. Oxford, England. John Wiley & Sons 31-41
- Pukall CF, Binik YM, Khalife S, et al. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96(1-2: 163-75.
- Arnold, LD., Bachmann, G.A., Rosen, R., Kelly, S., & Rhoads, G.G. Vulvodynia: Characteristics and associations with comorbidities and quality of life. Obstetrics & Gynecology. 2006; 107:617–624.
- Gunter, J. Vulvodynia: New thoughts on a devastating condition. Obstetrical & Gynecology Survey, 2007;62, 812–819.
- Kennedy, C.M., Nygaard, I.E., Bradley, C.S., Galask, R.P. Bladder and bowel symptoms among women with vulvar disease: Are they universal? Journal of Reproductive Medicine. 2007;52, 1073–1078.
- Peters, K., Girdler, B., Carrico, D., Ibrahim, I., Diokno, A. Painful bladder syndrome/interstitial cystitis and vulvodynia: A clinical correlation. International Urogynecology Journal and Pelvic Floor Dysfunction. 2007; 19: 665–669
- Pukall CF, Baron M, Amsel R, et al. Tender point examination in women with vulvar vestibulitis syndrome. Clin.J.Pain. 2006; 22(7) 601-9
- Clauw DJ, Schmidt M, Radulovic D, Singer A, Katz P, Bresette J. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125–3.
- Meeus M, Nijs J, Van de Wauwer N, Toeback L, Truijen S. Diffuse noxious inhibitory control is delayed in chronic fatigue syndrome: an experimental study. Pain. 2008;139:439–48
- Giesecke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–23.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain 2013; 14:579–589.
- Farmer MA. What is special about the vulvar vestibule? Pain. 2015 Mar;156(3):359-360. doi: 10.1097/j.pain.0000000000000094. PMID: 25687538; PMCID: PMC4335348.
- Falsetta M, Foster DC, Woeller CF, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015; 123(1):38e1-12.
- Fields HL, Basbaum AJ, Wall PD, Melzack R. Central nervous system mechanisms of pain modulation. In: Textbook of Pain, 4e, 1999. (eds. P.D. Wall and R. Mellzack), 309-329. London: Churchill Livingstone
- Schweinhardt P, Kuchinad A, Pukall CF, et al. Increased gray matter density in young women with chronic pain. Pain. 2008;140(3):411-19.
- Sutton KS, Yessick LR, Wild CJ, Chamberlain SM. Pukall S. Exploring the neural correlates of touch and pain in women with provoked vestibulodynia. International Association for the Study of Pain. 2020; May 161(5) 925-937.
- Pukall CF, Binik Y, Khalife S, et al. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002; 96(1-2):163-75.
- Granot M, Zimmer EZ, Friedman M, et al. Association between quantitative sensory testing, treatment choice, and subsequent pain reduction in vulvar vestibulitis syndrome. J Pain. 2004;5(4):226-232.
- Zolnoun, Hartmann K, Lamvu G, et al. A conceptual model for the pathophysiology of vulvar vestibulitis syndrome Obstet. Gynecol. Surv. 2006;61(6)395-401.
- Wesselmann U, Bonham AD, Foster D. Vulvodynia: current state of the biologic science. Pain. 2016;155:1696-1701.
- Foster DC, Dworkin RH, Wood RW. Effects of intradermal foot and forearm capsaicin injections in normal and vulvodynia-afflicted women. Pain. 2005;117:128-136.
- Granot M, Friedman M, Yarnitsky D, Tamir A, Zimmer Z. Primary and secondary vulvar vestibulitis syndrome: systemic pain perception and psychophysical characteristics. Am J Obstet Gynecol. 2004; 191:138-142.
- Bergeron S, Reed BD, Wesselmann U, Bohm Starke, N. Vulvodynia. Nature Reviews/Disease Primers. 2020;6:36.
- Morrison P, Parrotte K. Pelvic Floor Muscle Dysfunction and Structural Processes in Vulvodynia.In: Female Sexual Pain Disorders, 2nd ed. Goldstein AT, Pukall CF, Goldstein I. John Wiley & Sons, Hoboken N.J. 2021; 53-62.
- Reissing E, Brown C, Lord M, et al. J. Psychosom. Obstet. Gynecol. 2005.26(2):107-113.
- Lev-Sagie A, Witkin S. Recent advances in understanding provoked vulvodynia. F100Res; 2016; 5:2581.
- Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J.Sex.Med. 2016;(4):572-590.
- Thibault- Gagnon S, Morin M. Active and passive components of pelvic floor muscle tone in women with provoked vestibulodynia: a perspective based on a review of the literature. J. Sex. Med. 2015;12(11):2178-2189.
- Gyang A, Hartman M, Lamvu G. Musculoskeletal causes of chronic pelvic pain. Obstet. Gynecol.2013;121(3):645-650.]/efn_note]
The muscle dysfunction may evolve from a protective tightening reflex to avoid pain on penetration, or penetration that a woman fears. (One pelvic floor physical therapist pointed out that the pelvic floor is one of the first areas of the body to react (tighten) in fight or flight situations). Or, the PFM hypertonicity dysfunction along with a disorder in the kinetic chain, such as symphysis pubis dysfunction, lumbar spine disorders, coccydynia, hip disorders, and abdominal wall pain or sacro-iliac joint dysfunction can also be related to vulvodynia.241 Faubion S, Shuster L, Bharucha A. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin. Proc. 2012.87(2):187-193.
- .Wesselman U, Bonham A, Foster D. Vulvodynia: current state of the biological science. Pain. 2014 Sep: 155(9):1696-1701.
- Bergeron S. Rosen NO, Morin M. Genital pain in women: beyond interference with intercourse. Pain. 2011. 152:1223-1225.
- Gatchel RJ, Peng YR, Peters MI, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull, 2007. 133:581-624.
- Chisari, C, Chilcot, J. The experience of pain severity and pain interference in vulvodynia patients: The role of cognitive‐behavioral factors, psychological distress and fatigue. Journal of Psychosomatic Research. 2017 Feb.; 93, 83–89
- Santerre-Baillargeon M, Rosen NO, Steben M, Pâquet M, Macabena Perez R, Bergeron S. Does Self-compassion Benefit Couples Coping With Vulvodynia? Associations With Psychological, Sexual, and Relationship Adjustment. Clin J Pain. 2018 Jul;34(7):629-637. doi: 10.1097/AJP.0000000000000579. PMID: 29271798.
- Bois K , Bergeron S, Rosen N, McDuff P, Grégoire C. Sexual and relationship intimacy among women with provoked vestibulodynia and their partners: Associations with sexual satisfaction, sexual function, and pain self‐efficacy. The Journal of Sexual Medicine. 2013; 10:2024–2035.
- Chissari C, Monajemi M, Scott W, Moss-Morris R, McCracken L. Psychosocial factors associated with pain and sexual function in women with vulvodynia: A systematic review Eur J Pain. 2021;25:39–50
- Chisari C, Monajemi MB, Scott W, et al. Psychological factors associated with pain and sexual function in women with vulvodynia: A systematic review. Eur J Pain. 2021 Jan; 25(1): 39-50.
- Nguyen RH, Ecklund AM, MacLehose RF., Veasley C, and Harlow BL. Co-morbid pain conditions and feelings of invalidation and isolation among women with vulvodynia. Psychology, Health & Medicine. 2012. Vol. 17, No. 5, October, 589–598
- Singhai A, Tien K-Y, Hsai RY. Racial ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016. 11(8): e0159224.
- Maile DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vulvodynia: a controlled study. J Sex Med. 2015; 12:505-515.
- Ayling K and Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in heterosexual women. Arch Sex Behav. 2008; 37: 294-304.
- Gilbert R, et al. Burden and consequences of childhood maltreatment in high income countries. Lancet. 2009; 373:68-81.
- Godbout N, Dutton DG, Lussier Y, and Sabourin S. Early exposure to violence, domestic violence, attachment representations, and marital adjustment. Pers. Relat 2009;16:365-384.
- Griffin DW, Bartholomew K. Models of the self and other: fundamental dimensions underlying measures of adult attachment. J. Pers. Soc. Psychol. 1994; 67:430-445
- Ein-Dor T, Mikulincer M, Shaver PR. Attachment insecurities and the processing of threat related information: studying the schemas involved in insecure people’s coping strategies. J.Pers.Soc.Psychol.2011;101:78-93.
- Mikulincer M. Dolev T, Shaver PR. Attachment-related strategies during thought suppression: ironic rebounds and vulnerable self-representations. J. Pers. Soc. Psychol. 2004;87: 940-956.
- Berant E, Mikulincer M, Florian V. The association of mothers’ attachment style and their psychological reactions to the diagnosis of infant’s heart disease. J. Soc. Clin. Psychol. 2001;20: 208-232.
- Charbonneau-Lefebvre, Vaillancourt-Morel M, Rosen, NO, Steben M, Bergeron S. Attachment and Childhood Maltreatment as Moderators of Treatment Outcome in a Randomized Clinical Trial for Provoked Vestibulodynia. J Sex Med. 2022 Mar; 19(3): 479-495
- Bois K, Bergeron S, Rosen N, et al. Intimacy, sexual satisfaction and sexual distress in vulvodynia couples: an observational study. Health Psychol. 2016;35: 531-540.
- Dias-Amaral A, Marques-Pinto A. Female Genito-Pelvic Pain/Penetration Disorder: Review of the Related Factors and Overall Approach. Rev Bras Ginecol Obstet. 2018 Dec;40(12):787-793. English. doi: 10.1055/s-0038-1675805. Epub 2018 Nov 14. PMID: 30428492.
- Rosen NO, Bergeron S, Sadikaj G, Glowacka M, Delisle I, Baxter ML. Impact of male partner responses on sexual function in women with vulvodynia and their partners: a dyadic daily experience study. Health Psychol. 2014 Aug;33(8):823-31. doi: 10.1037/a0034550. Epub 2013 Nov 18. PMID: 24245835.
- Lemieux AJ, Bergeron S, Steben M, and Lambert B. Do romantic partners’ responses to entry dyspareunia affect women’s experience of pain? The roles of catastrophizing and self-efficacy. J. Sex. Med. 2013;10:2274-2284.
- Jodoin M, Bergeron S, Khalife S, et al. Male partners of women with provoked vestibulodynia: attributions for pain and their implications for dyadic adjustment, sexual satisfaction, and psychological distress. J Sex.Med. 2008;5:2862-2870.
- Davis SN, Bergeron S, Bois K, et al. A prospective 2 year examination of cognitive and behavioral correlates of provoked vestibulodynia. Clin.J.Pain. 2015;: 333-341.
- Bergeron S, Rosen NO. Psychosocial Factors in Vulvodynia. In: Female Sexual Pain Disorders, 2nd edition. Goldstein AT, Pukall CF, Goldstein I., Eds.Wiley Blackwell 2021
- Gates EA, Galask RP. Psychological and sexual functioning in women with vulvar vestibulitis. J. Psychosom. Obstet. Gynecol. 2001;22:221.
- Reed Bd, Haefner HK, Punch MR, et al. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. J. Reprod. Med. 2000;45:624.
- Meana M, Binik YM, Khalife S, et al. Biopychosocial profile of women with dyspareunia. Obstet. Gynecol 1997; 90:583.
- Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav, 2013;42:999.
- Singhmar P, et al. P116 is a non-neuronal regulator of neuropathic pain. Preprint at bioRxiv https:doi.org /10.1101/695429 (2019)
- Falsetta ML. et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015; 213: e12.
- Falsetta ML. et al. Toll-like receptor signaling contributes to proinflammatory mediator production in localized provoked vulvodynia. J Low. Genit. Tract Dis. 2018;22;52-57
- Foster DC, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia affected and pain free women. Pain. 2015;15:386396.
- Olivier M, Asmis R, et al. The Need for Multi-omics Biomarker Signatures in Precision Medicine. Int J Mol Sci. 2019 Oct; 20 (19)
- Labus JS, Mayer EA, Aagaard A, Stains J, Broniowska K, Rapkin A. Reduced concentrations of vaginal metabolites involved in steroid hormone biosynthesis are associated with increased vulvar vestibular pain and vaginal muscle tenderness in provoked vestibulodynia: An exploratory metabolomics study. Molecular Pain. 2021;17:1-15.
- Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett. 2012;520:197-203.
- Pukall CF, Strigo JA, Binik YM, Khalife S, Bushnell Mc. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115:118-27.
- Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. J. Pain. 2013;14: 579-89.
- Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young with chronic vulvar pain. Pain. 2008;411-9.
- Henzell H, Berzins K, Langford JP. Provoked vestibulodynia: current perspectives. Int J Womens Health 2017; 9: 631-642.
- Brotto LA, Sadownik LA, Thomson S, Dayan M, Smith KB, Seal BN, Moses M, Zhang A. A comparison of demographic and psychosexual characteristics of women with primary versus secondary provoked vestibulodynia. Clin J Pain. 2014 May;30(5):428-35. doi: 10.1097/AJP.0b013e31829ea118. PMID: 23887337.
- Goetsch, M. F., Morgan, T. K., Korcheva, VB, Li, H, Peters, D, & Leclair, CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: A prospective study. American Journal of Obstetrics and Gynecology 2010. 202(6), 614-614.e8.
- Leclair C, Goetsch MF. Differences in primary compared with secondary vestibulodynia by immunochemistry. Obstet Gynecol 2011 Jun; 117(6): 1307-1313.
- Aerts L, Bergeron S, Corsini-Munt S, Steben M, Pâquet M. Are primary and secondary provoked vestibulodynia two different entities? A comparison of pain, psychosocial, and sexual characteristics. J Sex Med. 2015 Jun;12(6):1463-73. doi: 10.1111/jsm.12907. Epub 2015 May 11. PMID: 25963291.
- Fontaine F, Dumoulin C, Bergeron S, Mayrand MH, Khalifé S, Wadell G, Morin M. Pelvic Floor Muscle Morphometry and Function in Women With Primary and Secondary Provoked Vestibulodynia. J Sex Med. 2018 Aug;15(8):1149-1157. doi: 10.1016/j.jsxm.2018.06.001. Epub 2018 Jul 20. PMID: 30033191.
- Oughourlian T, Tun G, et al. Symptom-associated alterations in functional connectivity in primary and secondary provoked vestibulodynia. PAIN. March 2023; 164(3): 653-665.
- Belanger C, Dumoulin C, Bergeron S, Mayrand MH, Khalifee S, Waddell G, Dubois MF, Morin M; PVD Group. Pain characteristics, fear-avoidance variables and pelvic floor function as predictors of treatment response to physical therapy in women with provoked vestibulodynia. Clin J Pain 2022;38:360–7.
- Burrows, Lara & Klingman, Daisy & Pukall, Caroline & Goldstein, Andrew. Umbilical hypersensitivity in women with primary vestibulodynia. Journal of reproductive medicine. 2008. 53. 413-6.
- Farmer MA. What is special about the vulvar vestibule? Pain. 2015 Mar;156(3):359-360. doi: 10.1097/j.pain.0000000000000094. PMID: 25687538; PMCID: PMC4335348.
- Falsetta, Megan L., et al. Specialized pro-resolving mediators reduce pro-nociceptive inflammatory mediator production in models of localized provoked vulvodynia. The journal of pain. 2021. 22.10: 1195-1209.
- Lev-Sagie A, Gilad R, Prus D. The vulvar vestibule, a small tissue with a central position: Anatomy, embryology, pain mechanisms, and hormonal associations. Current sexual health reports. 2019. 11:60-66. published online
- Foster DC. The Role of Inflammation in Vulvodynia. In: Female Sexual Pain Disorders, 2nd edition. Andrew Goldstein, Caroline Pukall, Irwin Goldstein, editors. John Wiley & Sons, 2021.
- Lev-Sagie A, Wertman O, Lavee Y, Granot M. Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse. J Clin Med. 2020. Jun 27;9(7):2023. doi: 10.3390/jcm9072023. PMID: 32605092; PMCID: PMC7409043.
- Farmer MA, Maykut CA, Huberman JS, Huang L, Khalifé S, Binik YM, Apkarian VA, Schweinhardt P. Psychophysical properties of female genital sensation. Pain. 2013 Nov;154(11):2277-2286. doi: 10.1016/j.pain.2013.05.028. Epub 2013 May 23. PMID: 23707679.
- Papoutsis D, Antonakou A. The Q-tip test of the vulva as a diagnostic aid for vulvodynia: sensitivity, specificity and predictive values. J Psychosom Obstet Gynaecol. 2019 Jun;40(2):90. doi:10.1080/0167482X.2017.1415882. Epub 2017 Dec 19. PMID: 29256294.
- Payne KA, Binik YM, Amsel R, Khalife S. When sex hurts, anxiety and fear orient attention towards pain. Eur J Pain. 2005;9:427-436.
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S; European Palliative Care Research Collaborative (EPCRC). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011 Jun;41(6):1073-93. doi: 10.1016/j.jpainsymman.2010.08.016. PMID: 21621130.
- Safikhani S, Gries KS, Trudeau JJ, Reasner D, Rüdell K, Coons SJ, Bush EN, Hanlon J, Abraham L, Vernon M. Response scale selection in adult pain measures: results from a literature review. J Patient Rep Outcomes. 2018 Sep 6;2:40. doi: 10.1186/s41687-018-0053-6. PMID: 30238085; PMCID: PMC6127068.
- Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health and Human Services. NIH Research plan on vulvodynia. Washington, D.C. United States Government Printing Office, 2012.
- Pukall CF, Young RA, Roberts MJ, Sutton KS, Smith KB. The vulvalgesiometer as a device to measure genital pressure-pain threshold. Physiol Meas. 2007 Dec;28(12):1543-50. doi: 10.1088/0967-3334/28/12/008. Epub 2007 Dec 3. PMID: 18057518.
- Foster DC, Beth Kotok M, Huang LS, Watts A, Oakes D, Howard FM, Stodgell CJ, Dworkin RH. The tampon test for vulvodynia treatment outcomes research: reliability, construct validity, and responsiveness. Obstet Gynecol. 2009 Apr;113(4):825-832. doi: 10.1097/AOG.0b013e31819bda7c. PMID: 19305326; PMCID: PMC2756618.
- Kaarbø MB, Danielsen KG, Haugstad GK, Helgesen ALO, Wojniusz S. The Tampon Test as a Primary Outcome Measure in Provoked Vestibulodynia: A Mixed Methods Study. J Sex Med. 2021 Jun;18(6):1083-1091. doi: 10.1016/j.jsxm.2021.03.010. Epub 2021 May 6. PMID: 33967000.
- Baguley SDK, Curnow JSH, Morrison GD, Barron LF. Vaginal algometer: development and application of a device to monitor vaginal wall pressure pain threshold Physiol. Meas. 2003;24:833–836.
- Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012 Sep;13(9):910-20. doi: 10.1016/j.jpain.2012.06.006. PMID: 22958875; PMCID: PMC3495612.
- Murina F, Barbieri S, Lubrano C, Cetin I. Vestibular Mucosa Thickness Measured by Ultrasound in Patients Affected by Vestibulodynia: A Case-Control Study. Sex Med. 2021 Apr;9(2):100320. doi: 10.1016/j.esxm.2020.100320. Epub 2021 Feb 13. PMID: 33588370; PMCID: PMC8072142.
- Shafik A. Pudendal canal syndrome as a cause of vulvodynia and its treatment by pudendal nerve decompression. Eur J Obstet Gynecol Reprod Biol. 1998;80:215–220.
- Popeney C, Ansell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: diagnosis and treatment. Neurourol Urodyn. 2007;26:820–827
- Labat JJ, Riant T, Robert R, et al. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27:306–310.
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p338-39.
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p333-347.
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p333.
- Arbel A. and Lev-Sagie A. Generalized Unprovoked Vulvodynia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p382.
- Wesselmann U., Bonham A., Foster DC. Vulvodynia: Current state of the biological science. Pain. 2014:Sep:155(9):1696-1701
- Faroud A,Alakassis A, Georges A, et al. Sexual dysfunction due to pudendal neuralgia: a systematic review. Transl Androl Urol.2021 Jun; 10(6): 2500–2511.
- Lev-Sagie A, Wertman O, Lavee Y, Granot M. Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse. J Clin Med. 2020 Jun 27;9(7):2023. doi: 10.3390/jcm9072023. PMID: 32605092; PMCID: PMC7409043.
- Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012 Sep;13(9):910-20. doi: 10.1016/j.jpain.2012.06.006. PMID: 22958875; PMCID: PMC3495612.
- Witzeman K, Nguyen RH, Eanes A, As-Sanie S, Zolnoun D. Mucosal versus muscle pain sensitivity in provoked vestibulodynia. J Pain Res. 2015 Aug 12;8:549-55. doi: 10.2147/JPR.S85705. PMID: 26316805; PMCID: PMC4540214
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Stav K, Dwyer PL, Roberts F, Roberts L. Pudendal neuralgia fact or fiction? Obstet Gynecol Survey. 2009; 64(3):190-199
- Labat JJ, Riant T, Robert R, et al. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27: 306–310.
- Eserdag S, Kurban D, Kiseli M, Alan M, Alan Y. The histopathological results of vestibulectomy specimens in localized provoked vulvodynia in Turkey. Pan Afr Med J. 2020 Nov 24;37:267. doi: 10.11604/pamj.2020.37.267.21240. PMID: 33598081; PMCID: PMC7864262.
- Swash M, Snooks SJ. Motor nerve conduction studies of the pelvic floor innervation. In: Henry MM, Swash M, eds. Coloproctology and the Pelvic Floor. 2nd ed. London: Butterworth- Heinemann, 1992:196–206.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62 (12): 812–19.
- Jantos M. Vulvodynia: a psychophysiological profile based on electromyographic assessment. 2008 Mar;33(1):29-38. doi: 10.1007/s10484-008-9049-y. Epub 2008 Jan 24. PMID: 18214669.
- Ciszek BP, Khan AA, Dang H, Slade GD, Smith S, Bair E, Maixner W, Zolnoun D, Nackley AG. MicroRNA expression profiles differentiate chronic pain condition subtypes. Transl Res. 2015 Dec;166(6):706-720.e11. doi: 10.1016/j.trsl.2015.06.008. Epub 2015 Jun 24. PMID: 26166255; PMCID: PMC4656098.
- Cannon W. B. (1963). The Wisdom of the Body, Revised and Enlarged edition (first published 1939). New York, NY: W.W. Norton & Co., Inc.
- Selye Hans. The General Adaptation Syndrome and the Diseases of Adaptation. Journal of Clinical Endocrinology. 1946;6(no. 2):119–131.
- Jackson M.Chapter 1: Evaluating the Role of Hans Selye in the Modern History of Stress in Stress, Shock, and Adaptation in the Twentieth Century. 2014 Feb. Cantor D, Ramsden E, editors.Rochester (NY): University of Rochester Press.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977 Apr 8;196(4286):129-36. doi: 10.1126/science.847460. PMID: 847460.
- https://en.wikipedia.org/wiki/Biopsychosocial_model[/efn_note
- Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med. 2004 Nov-Dec;2(6):576-82. doi: 10.1370/afm.245. PMID: 15576544; PMCID: PMC1466742. In addition, current research is demonstrating, at a deeper physiological level, the interplays between the immune and nervous systems and their influence on cognition, behavior, psychiatric illness, and neurodegenerative disease, including in the context of chronic pain conditions.335Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Schiller, M., Ben-Shaanan, T.L. & Rolls, A. Neuronal regulation of immunity: why, how and where?. Nat Rev Immunol 2021.21, 20–36. https://doi.org/10.1038/s41577-020-0387-1
- Su PP, Zhang L, He L, Zhao N, Guan Z. The Role of Neuro-Immune Interactions in Chronic Pain: Implications for Clinical Practice. J Pain Res. 2022 Aug 4;15:2223-2248. doi: 10.2147/JPR.S246883. PMID: 35957964; PMCID: PMC9359791.
- Meade E, Garvey M. The Role of Neuro-Immune Interaction in Chronic Pain Conditions; Functional Somatic Syndrome, Neurogenic Inflammation, and Peripheral Neuropathy. Int J Mol Sci. 2022 Aug 2;23(15):8574. doi: 10.3390/ijms23158574. PMID: 35955708; PMCID: PMC9369187.
- Billman GE. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front Physiol. 2020 Mar 10;11:200. doi: 10.3389/fphys.2020.00200. PMID: 32210840; PMCID: PMC7076167.
- Lu S, Wei F, Li G. The evolution of the concept of stress and the framework of the stress system. Cell Stress. 2021 Apr 26;5(6):76-85 doi: 10.15698/cst2021.06.250. PMID: 34124582; PMCID: PMC8166217.
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p333.
- Sadownik LA, Yong PJ, Smith KB. Systematic Review of Treatment Outcome Measures for Vulvodynia. J Low Genit Tract Dis. 2018 Jul;22(3):251-259. doi: 10.1097/LGT.0000000000000406. PMID: 29933290.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Bohm-Starke N, Ramsay KW, Lytsy P, Nordgren B, Sjöberg I, Moberg K, Flink I. Treatment of Provoked Vulvodynia: A Systematic Review. J Sex Med. 2022 May;19(5):789-808. doi: 10.1016/j.jsxm.2022.02.008. Epub 2022 Mar 21. PMID: 35331660.
- Lamvu G, Alappattu M, Witzeman K, Bishop M, Robinson M, Rapkin A. Patterns in Vulvodynia Treatments and 6-Month Outcomes for Women Enrolled in the National Vulvodynia Registry-An Exploratory Prospective Study. J Sex Med. 2018 May;15(5):705-715. doi: 10.1016/j.jsxm.2018.03.003. Epub 2018 Apr 7. PMID: 29631955; PMCID: PMC6613576.
- Foster DC, Kotok MB, Huang LS, Watts A, Oakes D, Howard FM, Poleshuck EL, Stodgell CJ, Dworkin RH. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010 Sep;116(3):583-593. doi: 10.1097/AOG.0b013e3181e9e0ab. PMID: 20733439; PMCID: PMC6545923.
- Goldstein AT, Pukall VF, Brown C, Bergeron S, Stein A, Kellog-Spadt. Vulvodynia: Assessment and Treatment. J. Sex. Med. 2016;13:572-590.
- Rosen NO, Dawson SJ, Brooks M, Kellogg-Spadt S. Treatment of Vulvodynia: Pharmacological and Non-Pharmacological Approaches. Drugs. 2019 Apr;79(5):483-493. doi: 10.1007/s40265-019-01085-1. PMID:30847806.
- Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J Sex Med 2012;9:2077-92
- Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J Sex Med 2012;9:2077-92
- Goldstein AT, Pukall VF, Brown C, Bergeron S, Stein A, Kellog-Spadt. Vulvodynia: Assessment and Treatment. J. Sex. Med. 2016;13:572-590.
- Chisari C, Monajemi MB, Scott W, Moss-Morris R, McCracken LM. Psychosocial factors associated with pain and sexual function in women with Vulvodynia: A systematic review. Eur J Pain. 2021 Jan;25(1):39-50. doi: 10.1002/ejp.1668. Epub 2020 Oct 19. PMID: 33001545; PMCID: PMC7821117.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Sarkhel S, Singh OP, Arora M. Clinical Practice Guidelines for Psychoeducation in Psychiatric Disorders General Principles of Psychoeducation. Indian J Psychiatry. 2020 Jan;62(Suppl 2):S319-S323. doi: 10.4103/psychiatry. Indian J Psychiatry._780_19. Epub 2020 Jan 17. PMID: 32055073; PMCID: PMC7001357
- https://my.clevelandclinic.org/health/treatments/21208-cognitive-behavioral-therapy-cbt
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- McRae, K., & Gross, J. J. 2020. Emotion regulation. Emotion, 20(1), 1–9. https://doi.org/10.1037/emo0000703
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Bergeron S. et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic feedback, and vestibulectomy in the treatment of vulvar vestibulitis. Pain. 2001;91:297-306.
- Bergeron S, Khalife S, Glazer H, Binik YM. Surgical and behavioral treatments for vestibulodynia: two and one half year follow-up and predictors of outcome. Obstet Gynecol. 2008;111:159-165.
- Pukall CF, et al. Vulvodynia definition prevalence, impact, and pathophysiological factors. J. Sex. Med. 2016;13:291-304.
- Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: cognitive behavioral clinical trial for women with vulvodynia; cognitive behavioral therapy vs. supportive therapy. Pain. 2009;141:31-40.
- Corsini Munt S, Bergeron S, Rosen NO, Mayrand NH, Delisle I. Feasibility and preliminary effectiveness of a novel cognitive-behavioral couple therapy for provoked vestibulodynia. J.Sex. Med. 2014;11:2515-2527.
- Brotto LA, Basson R, Smith KB, Driscoll M, Sadownik L. Mindfulness-based group therapy for women with provoked vestibulodynia. Mindfulness. 2015;6: 417-432.
- Brotto LA, et al. A comparison of mindfulness-based cognitive therapy in a hospital clinic setting. J. Sex. Med. 2019; 16: 9909-923.
- Brotto LA, Bergeron S, Zdniuk B, Basson R. Mindfulness and cognitive behavior therapy for provoked vestibulodynia: mediators of treatment outcome and long-term effects. J. Consult Clin. Psychol. 2020; 88:48-64.
- Falsetta ML, Foster DC, Bonham AD, Phipps RP. A review of the available clinical therapies for vulvodynia management and new data implicating proinflammatory mediators in pain elicitation. BJOG. 2016;124(2); 210-218.
- Hartmann D, Strauhal MJ, Nelson CA. Treatment of women in the United States with localized, provoked vulvodynia:practice survey of women’s health physical therapists. J. Reprod. Med. 20007;52; 48-52.
- Bergeron S, Rosen N, Morin M. Genital pain in women: beyond interference with intercourse. Pain. 2011. 152; 1225.
- Morin M, Carroll MS, Bergeron S. Systematic review of the effectiveness of physical therapy modalities in women with provoked vestibulodynia. Sex. Med. Rev. 2017;5:295-322.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Shrikhande A, Ullger C, Seko K, Patil S, Natarajan J, Tailor Y, Thompson-Chudy C. A physiatrist’s understanding and application of the current literature on chronic pelvic pain: a narrative review. Pain Rep. 2021 Aug 30;6(3):e949. doi: 10.1097/PR9.0000000000000949. PMID: 34476302; PMCID: PMC8407606.
- https://www.mayoclinic.org/diseases-conditions/myofascial-pain-syndrome/
- Ajimsha MS, Al-Mudahka NR, Al-Madzhar JA. Effectiveness of myofascial release: systematic review of randomized controlled trials. J Body Mov Ther. 2015 Jan;19(1):102-12. doi: 10.1016/j.jbmt.2014.06.001. Epub 2014 Jun 13. PMID: 25603749.
- Grinberg K, Weissman-Fogel I, Lowenstein L, Abramov L, Granot M. How Does Myofascial Physical Therapy Attenuate Pain in Chronic Pelvic Pain Syndrome? Pain Res Manag. 2019 Dec 12;2019:6091257. doi: 10.1155/2019/6091257. PMID: 31915499; PMCID: PMC6930783.
- Wong CS, Wong SH. A new look at trigger point injections. Anesthesiol Res Pract. 2012;2012:492452.
- Wong CS, Wong SH. A new look at trigger point injections. Anesthesiol Res Pract. 2012;2012:492452.
- Wong CS, Wong SH. A new look at trigger point injections. Anesthesiol Res Pract. 2012;2012:492452.
- Langford CF, Nagy SU, and Ghoniem GM. Levator ani trigger point injections: an under utilized treatment for chronic pelvic pain. Neurourology and Urodynamics. 2007. vol. 26, no. 1, pp. 59–62.
- Morin M, Carroll MS, Bergeron S. Systematic review of the effectiveness of physical therapy modalities in women with provoked vestibulodynia. Sex. Med. Rev. 2017;5:295-322.
- Bergeron S, Khalife S, Glazer H, Binik YM. Surgical and behavioral treatments for vestibulodynia: two and one half year follow-up and predictors of outcome. Obstet Gynecol. 2008;111:159-165.
- Morin M, et al. Efficacy of multimodal physiotherapy treatment compared to overnight topical lidocaine in women with provoked vestibulodynia: a bi-center randomized controlled trial. J.Sex.Med. 2016;13:S243.
- Morin M, Carroll MS, Bergeron S. Systematic Review of the Effectiveness of Physical Therapy Modalities in Women With Provoked Vestibulodynia. Sex Med Rev. 2017 Jul;5(3):295-322. doi: 10.1016/j.sxmr.2017.02.003. Epub 2017 Mar 28. PMID: 28363763.
- Munday P, Buchan A, Ravenhill F, et al. A qualitative study of women with vulvodynia II. Response to a multidisciplinary approach to management. J. Reprod. Med. 2007;52:19-22.
- Sadownik LA, Seal BN, Brotto LA. Provoked vestibulodynia-women’s experience of participating in a multidisciplinary program. J Sex Med. 2012;9:1086-1093.
- Kaarbø MB, Danielsen KG, Helgesen ALO, Wojniusz S, Haugstad GK. A conceptual model for managing sexual pain with somatocognitive therapy in women with provoked vestibulodynia and implications for physiotherapy practice. Physiother Theory Pract. 2022 Jul 10:1-14. doi: 10.1080/09593985.2022.2096516. Epub ahead of print. PMID: 35815605.
- Swanson AL. Vulvodynia: diagnosis and management. Obstet Gynecol Clin of North Am 2017;44:493-508.
- Woodruff JD, Surgery of the vulva. TeLinde”s Operative Gynecology, 5th ed. RF Mattingly, ed. Philadelphia, Lippincott, 1976.
- Woodruff JD. Genadry R, Poliakoff S. Treatment of dyspareunia and vaginal outlet distortions by perineoplasty. Obstet Gynecol. 1982 Jun; 57 (6): 750-4.
- Woodruff JD and Parmley TH. Minor Vestibular Gland Infection. Obstet Gynecol 1983; 62(5): 609-612.
- Goldstein A. Perineoplasty and Vaginal Advancement Flap for Vulvar Granuloma Fissuratum. J Sex Med.2011; 8(11): 2984-2987.
- Goldstein A. Perineoplasty and Vaginal Advancement Flap for Vulvar granuloma Fissuratum. J Sex Med. 2011; 8(11): 2984-2987
- Tammola P. Surgical treatment of vulvar vestibulitis: a review. Acta Obstetrica et Gynaecologica Scandinavia; 2010;89 (11); 1385-1395.
- Goetsch MF. Simplified surgical revision of the vulvar vestibule for vulvar vestibulitis. Am J Obstet Gynecol. 1996;174:1701-7.
- Sadownik L. Etiology, diagnosis and clinical management of vulvodynia. Int J Women’s Health 2014. 6: .437-449.
- Tammola P. Surgical treatment of vulvar vestibulitis: a review. Acta Obstetrica et Gynaecologica Scandinavia; 2010;89 (11); 1385-1395.
- Rosen NO, Dawson SJ, Brooks M, Kellog-Spadt S. Treatment of Vulvodynia: Pharmacological and Non-Pharmacological Approaches. Drugs. 2019; 79: 483-493.
- Bergeron S, Reed B, Wesselmann U, Bohm-Starke N. Vulvodynia. Nature Reviews Disease Primers; 2020; 6:76.
- Tammola P. Surgical treatment of vulvar vestibulitis: a review. Acta Obstetrica et Gynaecologica Scandinavia; 2010;89 (11); 1385-1395.
- Das D, Davidson EW, Walters M, Farrell R., Ferrando CA. Patient-Centered Outcomes After Modified Vestibulectomy. Obstet Gynecol.2020;135(1):113-121.
- Bergeron S, Khalife S, Glazer H, Binik Y. Surgical and behavioral treatments for vestibulodynia: two and one half year follow-up and predictor of outcome. Obstet Gynecol. 2008; 111: 159-166.
- Bergeron S,Reed B, Wesselmann U, Bohne-Starke N. Vulvodynia. Nature Reviews/Disease Primers. 2020; 6:36.
- Bohm-Starke N, Ramsay KW, Lytsy P, Nordgren B, Sjöberg I, Moberg K, Flink I. Treatment of Provoked Vulvodynia: A Systematic Review. J Sex Med. 2022
- Farmer M, Maycut CA, et al. Psychophysical properties of female genital sensation. P 2013 Nov:154(11): 2277-2286.
- Lev-Sagie A, et al. Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse. J Clin Med 2020 Jun 27;9 (7): 2023.
- Velikonja L, Giovannetti O, Adams MA, Tomalty D. Innervation of the human vulvar vestibule: A comprehensive review. Clinical Anatomy. 2023; 36: 18-27,
- Falsetta M.L., Foster D.C., Bonham A.D., Phipps R.P. A review of the available clinical therapies for vulvodynia management and new data implicating proinflammatory mediators in pain elicitation. BJOG. 2017;124:210–218. doi: 10.1111/1471-0528.14157
- Falsetta ML, Wood RW, Linder MA, Bonham AD, Honn KV, Maddipati KR, Phipps RP, Haidaris CG, Foster DC. Specialized Pro-resolving Mediators Reduce Pro-nociceptive Inflammatory Mediator Production in Models of Localized Provoked Vulvodynia. J Pain. 2021 Oct;22(10):1195-1209. doi: 10.1016/j.jpain.2021.03.144. Epub 2021 Apr 1. PMID: 33813057; PMCID: PMC8484336.
- Reed BD, Haefner HK, Edwards L. A survey on diagnosis and treatment of vulvodynia among vulvodynia researchers and members of the International Society for the Study of Vulvovaginal Disease. J Reprod Med. 2008 Dec;53(12):921-9. PMID: 19160650.
- Bergeron S, Reed BD, Wesselmann U, Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. 2020 Apr 30;6(1):36. doi: 10.1038/s41572-020-0164-2. PMID: 32355269.
- Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003 Jul;102(1):84-7. doi: 10.1016/s0029-7844(03)00368-5. PMID: 12850611
- Morin M, Dumoulin C, Bergeron S, Mayrand MH, Khalifé S, Waddell G, Dubois MF; PVD Study Group. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021 Feb;224(2):189.e1-189.e12. doi: 10.1016/j.ajog.2020.08.038. Epub 2020 Aug 18. PMID: 32818475.
- Baron R. Capsaicin and nociception: from basic mechanism to novel drug. Lancet. 2000; 356:785-786.
- Murina F, Radici G, Bianco V. Capsaicin and the treatment of vulvar vestibulitis syndrome: a valuable alternative? MedGenMed. 2004 Dec 8;6(4):48. PMID: 15775875; PMCID: PMC1480562.
- Steinberg AC, Oyama IA, Rejba AE, Kellogg-Spadt S, Whitmore KE. Capsaicin for the treatment of vulvar vestibulitis. Am J Obstet Gynecol. 2005 May;192(5):1549-53. doi: 10.1016/j.ajog.2004.10.626. PMID: 15902156.
- R. Akel, C. E. Cohen, C. Fuller, Caution with topical capsaicin, Clinical and Experimental Dermatology, Volume 45, Issue 6, 1 August 2020, Page 739, https://doi.org/10.1111/ced.14226
- Bergeron S, Reed, BD, Wesselmann U, Bohm-Starke N. Vulvodynia. 2020:6:36.
- Bergeron S, Khalife S, Dupuis MJ, McDuff PA. A randomized clinical trial comparing group cognitive behavioral therapy and a topical steroid for women with dyspareunia. J. Consult.Clin. Psychol. 2016; 84:259-268,
- Goldstein AT, Pukall CF, Brown C, Bergeron S, Stein A, Kellogg-Spadt S. Vulvodynia: Assessment and Treatment. J Sex Med. 2016 Apr;13(4):572-90. doi: 10.1016/j.jsxm.2016.01.020. Epub 2016 Mar 25. PMID: 27045258.
- Murina F, Tassan P, Roberti P, Bianco V. Treatment of vulvar vestibulitis with submucous infiltrations of methylprednisone and lidocaine. An alternate approach. J. Reprod. Med. 2001;46:713-716.
- Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25:491-502.
- Bornstein J, Cohen Y, Zarfati D, et al. Involvement of heparinase in the pathogenesis of localized vulvodynia. Int J Gynecol Pathol. 2008;27:136-141.
- Goldstein AT, Pukall CF, Brown C, Bergeron S, Stein A, Kellogg-Spadt S. Vulvodynia: Assessment and Treatment. J.Sex. Med. 2016; 13:572-590.
- Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genital Tract Dis. 2012;16: 427-436.
- Farajun Y, Zarfati D, Abramov L, et al. Enoxaparin treatment for vulvodynia: a randomized controlled trial. Obstet Gynecol . 2012;120: 565-572.
- Brown CS, Wan J, Bachman G, Rosen R. Self-management, amitriptyline, and amitriptyline plus triamcinolone in the management of vulvodynia. J Women Health (Larchmt). 2009; 18(2):163-169.
- Brown CS, Wan J, Bachman G, Rosen R. Self-management, amitriptyline, and amitriptyline plus triamcinolone in the management of vulvodynia. J Women Health (Larchmt). 2009; 18(2):163-169.
- Reed BD, Caron AM, Gorenflo DW, Haefner HK. Treatment of vulvodynia with tricyclic antidepressants: efficacy and associated factors. J Low Genit Tract Dis. 2006; 10(4):245-25
- Eppsteiner E, Boardman L. Stockdale C. Vulvodynia. Best Practice Res Clin Obstet Gynaecol 2014;28: 100-12.
- Dharmshaktu P, Tayal V, Kalra BS. Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol. 2012 Jan;52(1):6-17.
- Foster DC, Kotok MB, Huang LS, Watts A, Oakes D, Howard FM, Poleshuck EL, Stodgell CJ, Dworkin RH. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010 Sep;116(3):583-593. doi: 10.1097/AOG.0b013e3181e9e0ab. PMID: 20733439; PMCID: PMC6545923.
- Pagano R, Wong S. Use of amitriptyline cream in the management of entry dyspareunia due to provoked vestibulodynia. J Low Genit Tract Dis. 2012 Oct;16(4):394-7. doi: 10.1097/LGT.0b013e3182449bd6. PMID: 22622338.
- Leo, RJA. A systematic review of the utility of anticonvulsant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013; 10: 2000-2008.
- Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia, unprovoked. J Reprod Med. 2007 Feb;52(2):103-6. PMID: 17393770.
- Brown C, Bachmann GA, Wan J, Foster GC, Gabapentin (GABA) Study Group. Gabapentin for the treatment of vulvodynia: a randomized controlled trial. Obstet Gynecol. 2018; 131: 1000-1007.
- Bachmann GA, et al. Effect of gabapentin on sexual function. In Vulvodynia: a randomized placebo-controlled trial. Am J Obstet Gynecol 2019;220:89e1-89e8.
- Goldstein AT, Belkin Zr, Krapf JM, et al. Polymorphisms of the androgen receptor gene and hormonal contraceptive induced provoked vestibulodynia. J. Sex. Med. 2014;11:2764.
- Burrows LJ, Basha M, Goldstein AT. The effects of hormonal contraceptives on female sexuality: a review. J Sex Med 2012;9:213.
- Johannesson U, de Broussard, Jansen GB, et al. Evidence of diffuse noxious inhibitory controls (DNIC) elicited by cold noxious stimulation in patients with provoked vestibulodynia. Pain. 2007;130:31.
- Bohm-Starke N, Johannesson U, Hilliges M, et al. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med 2004;49:888.
- Battaglia C, Morotti E, Persico N, et al. Clitoral vascularization and sexual behavior in young patients treated with drospirenone-ethinyl estradiol or contraceptive vaginal ring: a prospective randomized pilot study. J. Sex. Med. 2014;11:471
- Bazin S, Bouchard C, Brisson J, et al. Vulvar vestibulitis syndrome: an exploratory case control study .Obstet.Gynecol. 1994;83:47
- Bouchard C, Brisson J, Fortier M, et al. Use of oral contraceptive pills and vulvar vestibulitis : a case control study. Am J. Epidemiol 2002;156:254.
- Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med 2008; 53;102.
- Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J. Sex. Med. 2007;4:1679.
- Burrows LJ, Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex. Med. 2013;1:30.
- Casado-Espada NM, de Alarcón R, de la Iglesia-Larrad JI, Bote-Bonaechea B, Montejo ÁL. Hormonal Contraceptives, Female Sexual Dysfunction, and Managing Strategies: A Review. J Clin Med. 2019 Jun 25;8(6):908. doi: 10.3390/jcm8060908. PMID: 31242625; PMCID: PMC6617135.
- Reed BD, Harlow SD, Legocki LJ, et al. Oral contraceptive use and risk of vulvodynia: a population based longitudinal study. BJOG. 2013;120(13):1678-1684.
- Hong DG, Hwang SM, Park JM. Efficacy of ganglion impar block on vulvodynia: Case series and results of mid- and long-term follow-up. Medicine (Baltimore). 2021 Jul 30;100(30):e26799. doi: 10.1097/MD.0000000000026799. PMID: 34397737; PMCID: PMC8322564.
- Gunduz OH, Kenis-Coskun O. Ganglion blocks as a treatment of pain: current perspectives. J Pain Res 2017;10:2815–26.
- Rapkin AJ, McDonald JS, Morgan M. Multilevel local anesthetic nerve blockade for the treatment of vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2008; 198:41.e1
- McDonald JS and Rapkin AJ. Multilevel local anesthetic nerve blockade for the treatment of generalized vulvodynia: A pilot study. J Sex Med 2012;9:2919–2926.
- Weinschenk S, Benrath J, Kessler E, Strowitzki T, Feisst M. Therapy With Local Anesthetics to Treat Vulvodynia. A Pilot Study. Sex Med. 2022 Apr;10(2):100482. doi: 10.1016/j.esxm.2021.100482. Epub 2022 Jan 18. PMID: 35063914; PMCID: PMC9023246.
- Shrikhande A, Ullger C, Seko K, Patil S, Natarajan J, Tailor Y, Thompson-Chudy C. A physiatrist’s understanding and application of the current literature on chronic pelvic pain: a narrative review. Pain Rep. 2021 Aug 30;6(3):e949. doi: 10.1097/PR9.0000000000000949. PMID: 34476302; PMCID: PMC8407606.
- Rey Novoa M, Muñoz-Sellart M, Catalán Soriano M, Vinyes D. Treatment of Localized Vulvar Pain with Neural Therapy: A Case Series and Literature Review. Complement Med Res. 2021;28(6):571-577. English. doi: 10.1159/000514945. Epub 2021 Apr 12. PMID: 33845481
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p 339.
- Cain, Abigail MD, MPH; Carter, Kimberly MD, MPP; Salazar, Christina MD; Young, Amy MD. When and How to Utilize Pudendal Nerve Blocks for Treatment of Pudendal Neuralgia. Clinical Obstetrics and Gynecology 65(4):p 686-698, December 2022. | DOI: 10.1097/GRF.0000000000000715
- Cain, Abigail MD, MPH; Carter, Kimberly MD, MPP; Salazar, Christina MD; Young, Amy MD. When and How to Utilize Pudendal Nerve Blocks for Treatment of Pudendal Neuralgia. Clinical Obstetrics and Gynecology 65(4):p 686-698, December 2022. | DOI: 10.1097/GRF.0000000000000715
- Antolak S Jr, Antolak C, Lendway L. Measuring the Quality of Pudendal Nerve Perineural Injections. Pain Physician. 2016 May;19(4):299-306. PMID: 27228517.
- Peng PW, Tumber PS. Ultrasound-guided interventional procedures for patients with chronic pelvic pain – a description of techniques and review of literature. Pain Physician. 2008 Mar-Apr;11(2):215-24. PMID: 18354713.
- Kale A, Usta T, Basol G, Cam I, Yavuz M, Aytuluk HG. Comparison of Ultrasound-Guided Transgluteal and Finger-Guided Transvaginal Pudendal Nerve Block Techniques: Which One is More Effective? Int Neurourol J. 2019 Dec;23(4):310-320. doi: 10.5213/inj.1938112.056. Epub 2019 Dec 31. PMID: 31905278; PMCID: PMC6944786.
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p 339.
- Cain A, Carter K, Salazar C, Young A. When and How to Utilize Pudendal Nerve Blocks for Treatment of Pudendal Neuralgia. Clin Obstet Gynecol. 2022 Dec 1;65(4):686-698. doi: 10.1097/GRF.0000000000000715. Epub 2022 Jun 3. PMID: 35703212
- McDonald JS, Spigos DG. Computed tomography–guided pudendal block for treatment of pelvic pain due to pudendal neuropathy. Obstet Gynecol. 2000;95:306–309
- Filler A. Diagnosis and management of pudendal nerve entrapment syndromes: impact of MR neurography and open MR-guided injections. Neurosurg Q. 2008;18: 1–6
- Fanucci E, Manenti G, Ursone A, Fusco N, Mylonakou I, D’Urso S, Simonetti G. Role of interventional radiology in pudendal neuralgia: a description of techniques and review of the literature. Radiol Med. 2009 Apr;114(3):425-36. English, Italian. doi: 10.1007/s11547-009-0371-0. Epub 2009 Mar 10. PMID: 19277838.
- Labat JJ, Riant T, Lassaux A, Rioult B, Rabischong B, Khalfallah M, Volteau C, Leroi AM, Ploteau S. Adding corticosteroids to the pudendal nerve block for pudendal neuralgia: a randomised, double-blind, controlled trial. BJOG. 2017 Jan;124(2):251-260. doi: 10.1111/1471-0528.14222. Epub 2016 Jul 27. PMID: 27465823; PMCID: PMC5215631
- Marvel RP. Pudendal Neuralgia. In Female Sexual Pain Disorders, Evaluation and Management. Second edition. Goldstein, Pukall, and Goldstein editors. John Wiley and sons, 2021. p 339.
- Robert R, Labat J, Bensignor M, et al. Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long term evaluation. Eur Urol. 2005;47(3):403-408
- Hibner M, Desai N, Robertson LJ, Nour M. Pudendal neuralgia. J Minim Invasive Gynecol. 2010 Mar-Apr;17(2):148-53. doi: 10.1016/j.jmig.2009.11.003. Epub 2010 Jan 12. PMID: 20071246.
- Robert R, Labat JJ, Bensignor M, Pascal G, Deschamp C, Raoul S, Olivier H. Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long-term evaluation. European Urology. 2004;47(3):403-408
- Murer S, Polidori G, Beaumont F, Bogard F, Polidori É, Kinne M. Advances in the therapeutic approach of pudendal neuralgia: a systematic review. J Osteopath Med. 2021 Nov 22;122(1):1-13. doi: 10.1515/jom-2021-0119. PMID: 34800013.
- Chang MC. Efficacy of Pulsed Radiofrequency Stimulation in Patients with Peripheral Neuropathic Pain: A Narrative Review. Pain Physician. 2018 May;21(3):E225-E234. PMID: 29871378.
- Hamann W, Abou‐Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;10:171–176.
- Erdine S, Bilir A, Cosman E, et al. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009; 9:407-17.
- Rigaud J, Delavierre D, Sibert L, Labat J. Sympathetic nerve block in the management of chronic pelvic and perineal pain. Prog Urol. 2010; 20:1124-31
- Fang H, Zhang J, Yang Y, Ye L, Wang X. Clinical effect and safety of pulsed radiofrequency treatment for pudendal neuralgia: a prospective, randomized controlled clinical trial. J Pain Res. 2018 Oct 16;11:2367-2374. doi: 10.2147/JPR.S167866. PMID: 30410389; PMCID: PMC6200082.
- Krijnen EA, Schweitzer KJ, van Wijck AJM, Withagen MIJ. Pulsed Radiofrequency of Pudendal Nerve for Treatment in Patients with Pudendal Neuralgia. A Case Series with Long-Term Follow-Up. Pain Pract. 2021 Jul;21(6):703-707. doi: 10.1111/papr.12999. Epub 2021 Feb 22. PMID: 33522082; PMCID: PMC8359332.
- Krijnen EA, Schweitzer KJ, van Wijck AJM, Withagen MIJ. Pulsed Radiofrequency of Pudendal Nerve for Treatment in Patients with Pudendal Neuralgia. A Case Series with Long-Term Follow-Up. Pain Pract. 2021 Jul;21(6):703-707. doi: 10.1111/papr.12999. Epub 2021 Feb 22. PMID: 33522082; PMCID: PMC8359332.
- De Andres J, Sanchis-Lopez N, Asensio-Samper JM, Fabregat-Cid G, Villanueva-Perez VL, Monsalve Dolz V, Minguez A. Vulvodynia–An Evidence-Based Literature Review and Proposed Treatment Algorithm. Pain Pract. 2016 Feb;16(2):204-36. doi: 10.1111/papr.12274. Epub 2015 Jan 12. PMID: 25581081.
- Vallinga MS, Spoelstra SK, Hemel IL, van de Wiel HB, Weijmar Schultz WC. Transcutaneous electrical nerve stimulation as an additional treatment for women suffering from therapy-resistant provoked vestibulodynia: a feasibility study. J Sex Med. 2015 Jan;12(1):228-37. doi: 10.1111/jsm.12740. Epub 2014 Nov 12. PMID: 25388372.
- Dionisi B, Anglana F, Inghirami P, et al. Use of transcutaneous electrical stimulation and biofeedback for the treatment of vulvodynia (vulvar vestibular syndrome): result of 3 years of experience. Minerva Ginecol 2008; 60:485-91.
- Murina F, Bianco V, Radici G, Felice R, Di Martino M, Nicolini U. Transcutaneous electrical nerve stimulation to treat vestibulodynia: a randomized controlled trial. BJOG. 2008 Aug;115(9):1165-70. doi: 10.1111/j.1471-0528.2008.01803.x. PMID: 18715435.
- Vallinga MS, Spoelstra SK, Hemel ILM, et al.Transcutaneous Electrical Nerve Stimulation as an Additional Treatment for Women Suffering from Therapy-Resistant Provoked Vestibulodynia: A Feasibility Study. J Sex Med2015;12:228-37.
- https://www.ncbi.nlm.nih.gov/books/NBK537022/
- Larish AM, Dickson RR, Kudgus RA, et al. Vaginal Diazepam for Nonrelaxing Pelvic Floor Dysfunction: The Pharmacokinetic Profile. J Sex Med 2019;16;763-766.
- Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, Fariello JY, Whitmore KE. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010 Jul;21(7):895-9. doi: 10.1007/s00192-009-1075-7. Epub 2010 Jan 12. PMID: 20066399.
- Crisp CC, Vaccaro CM, Estanol MV, Oakley SH, Kleeman SD, Fellner AN, Pauls RN. Intra-vaginal diazepam for high-tone pelvic floor dysfunction: a randomized placebo-controlled trial. Int Urogynecol J. 2013 Nov;24(11):1915-23. doi: 10.1007/s00192-013-2108-9. Epub 2013 May 17. PMID: 23681047.
- Holland MA, Joyce JS, Brennaman LM, Drobnis EZ, Starr JA, Foster RT Sr. Intravaginal Diazepam for the Treatment of Pelvic Floor Hypertonic Disorder: A Double-Blind, Randomized, Placebo-Controlled Trial. Female Pelvic Med Reconstr Surg. 2019 Jan/Feb;25(1):76-81. doi: 10.1097/SPV.0000000000000514. PMID: 29280763.
- Stone RH, Abousaud M, Abousaud A, Kobak W. A Systematic Review of Intravaginal Diazepam for the Treatment of Pelvic Floor Hypertonic Disorder. J Clin Pharmacol. 2020 Dec;60 Suppl 2:S110-S120. doi: 10.1002/jcph.1775. PMID: 33274514
- Murina F, Felice R, Di Francesco S, et al.Vaginal diazepam plus transcutaneous electrical nerve stimulation to treat vestibulodynia: A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018; 228: 148-53.
- Murina F, Felice R, Di Francesco S, et al.Vaginal diazepam plus transcutaneous electrical nerve stimulation to treat vestibulodynia: A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol2018; 228: 148-53.
- Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004; 107:125-133.
- Park J, Chung ME. Botulinum toxin for Central Neuropathic Pain. Toxins 2018 April 10 (6): 224.
- Petersen CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A- a novel treatment for provoked vestibulodynia? Results from a randomized placebo controlled double blinded study. J. Sex. Med. 2009;6:2523-2537.
- Pelletier F, et al. Long term assessment of effectiveness and quality of life of OnabotulinumtoxinA injections in provoked vestibulodynia. J.Eur.Acad. Dermatol.Venerol. 2016;30:106-111.
- Diomande I. et al. Subcutaneous botulinum toxin type A injections for provoked vestibulodynia: a randomized placebo- controlled trial and exploratory subanalysis. Arch. Gynecol. Obstet. 2019;299: 993-1000.
- Brin MF, Vapnek JM. Treatment of vaginismus with botulinum toxin injections. Lancet. 1997;349:252–3.
- Jarvi SK, Abbott JA, Lenart MB, Steensma A, Vancaillie TG. Pilot study of botulintoxin type a in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Aust N Z J Obstet Gynaecol. 2004;44:46–50.
- Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-23.
- Karp B,Tandon H, Vigil D, Stratton P. Methodological approaches to botulinum toxin for the treatment of chronic pelvic pain, vaginismus, and vulvar pain disorders. International Urogynecology Journal. 2019. 30:1071–1081.
- Falsetta M. et al. specialized pro-resolving mediators reduce pro-nociceptive inflammatory mediator production in models of localized provoked vulvodynia. J Pain. 2021 Oce; 22 (10): 1195-1209.
- Falsetta M et al. Specialized pro-resolving mediators reduce pro-nociceptive inflammatory mediator production in models of localized provoked vulvodynia. J Pain. 2021 Oct;22(10):1195-1209.
- Falsetta ML, et al. Toll-like signaling contributes to proinflammatory mediator production in localized provoked vulvodynia. J. Low. Genit. Tract Dis. 2018;22: 52-57.
- Liao Z, et al. A local inflammatory renin-angiotensin system drives axon sprouting in provoked vestibulodynia. J. Pain.2017;18: 511-525.
- Chakrabarty A, Liao Z, Mu Y, Smith PG. Inflammatory renin-angiotensin system disruption attenuates sensory hyperinnervation and mechanical hypersensitivity in a rat model of provoked vestibulodynia. J Pain. 2018;19: 264-277.
- Falsetta M et al. Specialized pro-resolving mediators reduce pro-nociceptive inflammatory mediator production in models of localized provoked vulvodynia. J Pain. 2021 Oct;22(10):1195-1209.
- Foster DC, Falsetta ML, Woeller CF, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia-afflicted and pain-free women. www.painjournalonline 2015;156(3):386-396.
- Curran S, Brotto LA, Fisher H, Knudson G, Cohen T. The ACTIV study: acupuncture treatment in provoked vestibulodynia. J Sex Med. 2010 Feb;7(2 Pt 2):981-95. doi: 10.1111/j.1743-6109.2009.01582.x. Epub 2009 Nov 12. PMID: 19912491.
- Schlaeger JM, Xu N, Mejta CL, et al. Acupuncture for the treatment of vulvodynia: a randomized wait-list controlled pilot study. J. Sex. Med 2015;12:1019-1027.