Introduction
Hidradenitis suppurativa (HS), also known at acne inversa (AI), is a chronic, inflammatory skin condition typically involving the intertriginous skin of the axillary, groin, perianal, and inframammary regions. Clinical manifestations range from recurrent inflamed nodules and abscesses to draining sinus tracts and severe local scarring. The pain, malodor, drainage, and disfigurement from the disease can produce a profound negative impact on quality of life. Early lesions can mimic other disorders, and diagnosis is often delayed. Treatment goals include minimizing the risk of progression and avoiding disabling end-stage disease.
Epidemiology
The prevalence of HS varies by geographic reporting and is estimated to be between 0.1% and 4.1%. It is far more common in women than in men (3:1).1 Symptoms usually begin between puberty and age 40, with average age of onset between 20 and 30. It is rarely seen in children and the elderly. Familial cases indicate that genetic factors also play a role. Race or ethnicity may influence susceptibility to HS. In the United States, the prevalence is disproportionately high among blacks.2
Etiology
The etiology of HS is not fully understood, but is most likely multifactorial, involving genetic and environmental factors, lifestyle and diet, hormonal influences, immune and mechanical factors, and the local microbiota.
Broadly, the pathogenesis of HS involves follicular plugging, ductal rupture, and subsequent inflammation of the folliculopilosebaceous units (FPSUs), resulting in architectural tissue changes within affected skin. Ductal keratinocyte proliferation causes follicular hyperkeratosis and plugging. The plugged follicular duct expands, causing the production and release of antigens, cytokines, and other pro-inflammatory mediators, leading to a local perifolliculitis. If the follicular duct ruptures, the release of keratin fragments, hair, sebum, stem cells, and bacteria into the adjacent tissue recruits inflammatory cells, and the acute inflammatory response can evolve into a chronic foreign body-type granulomatous inflammation. Extruded stem cells can proliferate and create subcutaneous epithelialized skin tunnels which may interconnect, open to the skin, and become chronically inflamed.
A number of associated factors may contribute to the development or severity of HS:
Genetics: 40% of patients with HS will have an affected first-degree relative, and involvement seems to follow an autosomal dominant inheritance pattern. There are phenotypic and gender-specific variations in the expression of the disease, resulting in different patterns of involvement (axillary/mammary, follicular, and gluteal). Inheritance of abnormally formed folliculopilosebaceous units may explain the frequency of abnormal comedones in patients with HS.
Obesity and mechanical stress: HS is characteristically a disease of intertriginous areas of the body, where skin surfaces touch or rub together. Local skin maceration, heat, humidity, sweating, and friction can contribute to follicular plugging and follicular rupture. The process can also be exacerbated by friction from clothing along belt lines and under brassiere straps, and squeezing lesions to try and express contents can worsen local disease. Excess weight is more common in individuals with HS and, although the relationship between obesity and HS is complex, increasing BMI correlates positively with increasing severity of disease. Obesity increases levels of circulating pro-inflammatory cytokines and obese individuals with HS are at increased risk for hypertension, hypercholesterolemia, insulin resistance and diabetes, heart disease, stroke, and kidney disease.
Smoking: There is a strong association between smoking and HS (the majority of affected patients are either current or former smokers), and smokers tend to be more severely affected than non-smokers. Nicotine promotes dermal hyperplasia and induces follicular occlusion. Nicotine increases the production of IL-10, a cytokine involved in HS pathogenesis, and may influence bacterial propagation and biofilm formation.3 Nicotine can also increase local inflammation by inducing neutrophil chemotaxis and TNF-alpha production by keratinocytes.4
Hormones: The greater incidence of HS in women, typical onset after puberty, premenstrual flares, improvement during pregnancy, decrease in severity after menopause, more severe disease in men, and response of the disease to antiandrogen therapies implies a hormonal influence. However, studies have failed to consistently find higher rates of hyperandrogenism in HS cohorts compared to controls.5 Interestingly, disease tends to flare with contraceptive use of androgenic progestins like medroxyprogesterone acetate or levonorgestrel, and improve with oral contraceptives containing less androgenic progestins like drospirenone or cyproterone acetate (not available in the United States).
Diet: High gycemic carbohydrate diets, rich in dairy products, raise plasma glucose and insulin levels. Elevated insulin levels open androgen receptors, increasing local sensitivity to circulating androgens. Stimulation of follicular androgen receptors contributes to ductal keratinocyte overproduction and follicular plugging.6
Bacteria: Cultures from early, unruptured HS lesions are usually sterile. Older and ruptured lesions contain a wide variety of bacteria which are thought to be contaminants from normal skin flora (coagulase negative staphylococci, streptococci, Gram-negative rods, and anaerobic bacteria). Bacteria in HS lesions exacerbate the inflammatory response, and the bacterial biofilms may play a role in the persistence of inflammatory lesions.7 Microbiome differences between lesional and unaffected skin in HS patients versus healthy controls have been observed, and research into the pathologic dysbiosis is ongoing 8
Drugs: Lithium can trigger HS or cause it to flare. The above mentioned contraceptive use of androgenic progestins (levonorgestrel, medroxyprogesterone acetate) can also exacerbate existing disease.
Symptoms and clinical features
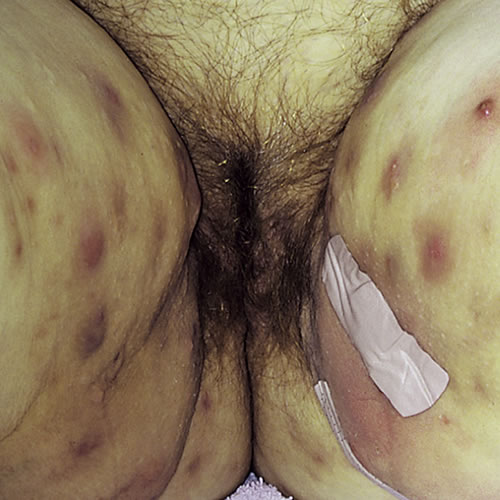
HS is primarily a disease of intertriginous areas. The axillary area is most commonly involved, but other typical areas include the inguinal area, inner thighs, perianal and perineal areas, mammary and inframammary regions, buttocks, pubic region, scrotum or vulva, trunk, and occasionally the scalp and retroauricular areas. Disease in women most commonly involves the groin, upper inner thigh, axilla, chest, buttocks, and intergluteal cleft. Areas of skin subjected to compression by clothing, such as belt lines, waistbands, abdominal folds, and brassiere straps are also common locations.
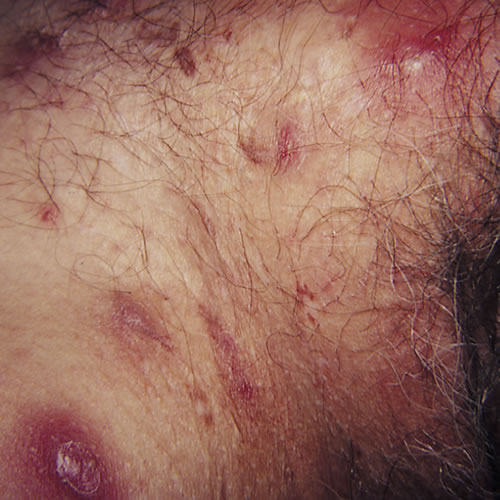
The initial lesions of HS are inflammatory nodules. They may be solitary, deep-seated, painful, 0.5 to 2 cm in diameter, with an insidious onset, and may last for several days to months. They tend to recur in the same location or the same skin region. Prodromal symptoms of fatigue and localized pruritus may be present. Because of the clinical similarity of HS nodules to furunculosis or “boils,” diagnosis of HS is frequently delayed, on average by seven years.
With persistence of disease, a nodule can progress to an abscess which may drain purulent or serosanguinous material to the skin surface, or nodules may regress without drainage over several weeks.
In longer-standing disease, multiple recurrent nodules within a limited area may form interconnecting sinus tracts, which can intermittently release blood stained, seropurulent, malodorous discharge. Ulceration and pyogenic granulomas can develop at sinus tract openings.
Comedones, often double or multi-headed, are typical of long standing disease. Scarring can occur in areas of active or healed disease. These scars can range from acneiform to dense fibrotic areas, thick plaques, or linear, rope-like bands. Bands over flexural areas can cause restriction of movement, and local tissue destruction can result in lymphatic obstruction and lymphedema.
Quality of life is often severely affected by HS. Pain can be severe, and should be proactively managed. Scarring, unpredictable discharge, and odor can result in social stigma, difficulty with employment, sexual dysfunction, low self-esteem, depression, anxiety, suicidal ideation, and completed suicides.
Diagnosis
There is no clinical or laboratory test for HS. Biopsy is often unhelpful. Diagnosis relies on:
Typical lesions: multiple deep nodules, abscesses, comedones, fibrosis, pitted scarring and bridged scarring, draining sinuses, and lymphedema.
Typical location of lesions: in axillae, groin, and inframammary areas most commonly. Lesions also occur in the perineal, perianal, buttocks, pubis vulva, chest, scalp, and post auricular locations.
Typical behavior of the lesions: long duration with little spontaneous regression, chronicity, and recurrences;9 cultures that are sterile or contain multiple species of bacteria; slow and incomplete resolution with antibiotics
A total body skin examination should be performed to assess the extent and severity of HS. There are three validated disease-severity evaluation tools: Hurley’s Clinical Staging, The Physician Global Assessment Tool for hidradenitis suppurativa (HS-PGA), and the International Hidradenitis Suppurativa Severity Score System (IHS4). The Sartorius System is detailed and complex, and is primarily used for research.
Hurley’s Clinical Staging10
Stage I
Abscess formation, single or multiple, without sinus tracts and cicatrization (scarring at the site of the wound).
Stage II
Recurrent abscesses with tract formation and cicatrization. Single or multiple, widely separated lesions with tunnel formation and/or scarring
Stage III
Diffuse or near-diffuse involvement, or multiple interconnected tracts and abscesses across entire area.
Physician Global Assessment Tool for hidradenitis:
Clear: No abscesses, draining tunnels, inflammatory nodules, or noninflammatory nodules.
Minimal: No abscesses, draining tunnels or inflammatory nodules, and the presence of noninflammatory nodules.
Mild: No abscesses or draining tunnels and 1-4 inflammatory nodules, or 1 abscess or draining tunnel and no inflammatory nodules.
Moderate: No abscesses or draining tunnels and >5 inflammatory nodules, or 1 abscess or draining tunnel and >1 inflammatory nodule, or 2-5 abscesses or draining tunnels and <10 inflammatory nodules.
Severe: 2-5 abscesses or draining tunnels and >10 inflammatory nodules.
Very Severe: >5 abscesses or draining tunnels.
International Hidradenitis Suppurativa Severity Score System (IHS4)
Mild: <3 points (number of inflammatory nodules X 1 and number of abscesses X 2)
Moderate: 4-10 points (number of inflammatory nodules X 1, number of abscesses X 2, and number of draining tunnels X 4).
Severe: >11 points (number of inflammatory nodules X 1, number of abscesses X 2, and number of draining tunnels X 4).
Pathology/Laboratory Findings
Histologic findings of hidradenitis suppurativa:
- Follicular hyperkeratosis with occlusion and dilatation of the hair follicle is seen in early lesions.
- Extensive perifolliculitis develops, and involvement of the apocrine gland and duct may or may not be apparent.
- Abscess formation occurs, leading to destruction of pilosebaceous units and, at times, other nearby adnexal structures, particularly apocrine glands.
- Sinus tracts and subcutaneous abscesses evolve with surrounding inflammation and extensive fibrosis.11
Unlike acne, significant sebaceous gland involvement is NOT a component of HS, either by histologic examination or by measuring the sebum secretion rate.12
ASSOCIATED DISORDERS AND SYNDROMES
Long term chronic inflammation can result in anemia, hypoproteinemia, amyloidosis, and infectious complications like lumbosacral abscess and sacral bacterial osteomyelitis.13
Metabolic syndrome (increased waist circumference, hypertriglyceridemia, low HDL cholesterol, increased blood pressure, increased fasting blood sugar) is very frequently present in patients with HS. Other comorbidities include chronic pulmonary disease and mild liver disease.14
Diabetes Type II prevalence is higher in HS than in the general population, with an incidence of 10.6-24.8% and patients with HS are twice as likely to develop Polycystic Ovary Syndrome compared with healthy controls.15
An association between HS and inflammatory bowel disease (Crohn disease (CD) and ulcerative colitis (UC)) has been suggested. In a recent meta-analysis, patients with HS had an increased odds ratio of having either Crohn disease or ulcerative colitis and patients with CD and UC were more likely to develop HS than the general public.16
HS is part of the follicular occlusion triad (HS, acne conglobata, dissecting cellulitis of the scalp, and pilonidal sinus), with follicular occlusion as the common event among these conditions. Acne vulgaris occurs more frequently in HS patients, and can be more severe and more difficult to treat in this population.
There is an increased prevalence of lymphoma (non-Hodgkin lymphoma, Hodgkin lymphoma, cutaneous T cell lymphoma) in patients with HS. Squamous cell carcinoma has been described, usually in the gluteal and perianal location, and more commonly in men. Human papilloma virus infection may contribute to the development of squamous cell carcinoma lesions in HS.17
Differential Diagnosis
Differential diagnosis includes bacterial infections (folliculitis, furuncles, carbuncles, abscesses), acne vulgaris, Bartholin cyst, pilonidal cyst, Crohn disease, lymphogranuloma venereum, granuloma inguinale, tuberculosis abscess, perirectal abscess, anal fistula, deep fungal infections, cat scratch disease, tularemia, pyoderma gangrenosum, and noduloulcerative syphilis.
There are seven questions to aid in differential diagnosis.18 Patients usually answer Yes to 1-4,19 No to 5-7. 20
1. Does anyone in your family have the same symptoms?
2. Do the boils recur in the same spots?
3. Do you smoke or use tobacco products?
4. Do your boils flare before your menstrual period?
5. Have the treatments you’ve received been helpful?
6. Do you get a fever with these boils?
7. Do you have infections elsewhere?
Treatment/management
Treatment of Hurley Stage II and II can be very difficult and requires a dermatologist. A surgeon will be needed for extensive disease.
For all patients:
Hope for HS: https://hopeforhs.org/
My HS Team: https://www.myhsteam.com/
Clinician to:
Perform a review of systems and physical exam to screen for tobacco use, obesity, hypertension, PCOS, inflammatory bowel disease, autoinflammatory disease, and inflammatory arthropathy. Obtain laboratory tests to screen for diabetes mellitus, hyperlipidemia, and anemia. Assess for comorbidities on a yearly basis, and support multidisciplinary involvement to manage comorbidities.
Control insulin resistance (metformin)
Treat both pain and inflammation with nonsteroidal anti-inflammatory drugs. Manage chronic pain proactively to avoid sleep disturbance, limitations of function, and psychological distress.
Screen for anxiety, depression, suicidal ideation, and manage appropriately.
Treat per Hurley’s stage or refer to an experienced colleague.
Patient to:
- Reduce heat, sweating, friction (weight loss, loose clothing, tampons, antiperspirants, avoid hot environments).
- Avoid any pressure and friction from panty hose, girdles, or other garments rubbing in the area. The use of ventilated cotton clothing (that is, cotton boxer shorts) is essential.
- The patient is instructed in gentle cleansing with triclosan solution once or twice a day. Topical 2% clindamycin in a mixture of isopropyl alcohol and propylene glycol twice a day is prescribed to avoid minor folliculitis. Bleach baths are helpful for large, draining, foul-smelling areas. (LINK, bleach bath).
- Stop smoking, nicotine avoidance
- Stop all dairy foods (casein, whey, milk solids); change to low glycemic-load diet. The diet is helpful to prevent new lesions and for efficacy of therapy for existing nodules. Dairy products contain multiple hormones and androgens that exacerbate HS.
Treatment: Hurley Stage I (Only a few flares yearly)
- Antibiotics are used for 7–10 days or longer for their anti-inflammatory effect:
Tetracycline 250-500 mg orally 4 x a day
Doxycycline 50 mg/d to 100 mg/d orally 2 x a day
Minocycline 100 mg orally 2 x a day
Erythromycin 1 gm orally, 1 x a day
Amoxicillin + Clavulinic acid 500 mg to1 gm orally every eight hours
Clindamycin 150-300 mg orally 2 x a day
Dicloxacillin 1-2 gm orally 2 x a day
Trimethaprim-sulfamethoxazole DS 1 tab orally 2 x a day - Zinc Gluconate 30-60 mg/d orally (anti-inflammatory, anti-androgenic)
- Avoiding incision and drainage is advisable since the abscesses almost invariably recur.1 For acute painful nodules an intralesional triamcinolone 10 mg/ml injection brings immediate relief. Shake vial well and withdraw 10 mg (one ml), then dilute with two ml saline or 0.25% Marcaine. Shake syringe well before injection. Inject 0.5 ml into center of painful lesions with 27-gauge needle.
- Oral contraceptives with ethinyl estradiol and low androgen-potential progestins such as ethynodiol diacetate or norethindrone + spironolactone 25-100 mg/day.
Treatment: Hurley Stage II (>1 flare/month or severe flares)
- Antibiotics are used for 7–10 days or longer for their anti-inflammatory effect:
Clindamycin 300 mg orally 2 x a day + Rifampin 300 mg orally 2 x a day x 3 months - Steroids:
Prednisone 40-60 mg orally x 3-4 days, then taper over 7-10 days
Intralesional Triamcinolone 5-10 mg/ml (0.1-0.5 ml) once monthly for 1 to 3 injections - Zinc Gluconate 30-60 mg/d (anti-inflammatory and anti-androgenic)
- Avlosulfone (Dapsone) 50-100 mg/d
- Anti-androgen treatment may be necessary. Newer oral contraceptives with desogestrel or norgestimate provide less androgenic effects. Spironolactone 100 to 150 mg a day can be added to these or to regular birth control pills for additional or long-term control. In Europe a truly androgen-blocking combination of ethinyl estradiol 35 μg and cyproterone acetate 2 mg (Diane 35) is available and is very effective. Finasteride 5 mg daily, a 5-alpha-reductase inhibitor which transforms testosterone into dihydrotestosterone is reported helpful. Due to possible feminization of a male fetus is case of pregnancy the drug must be prescribed with caution. Avoid Depot-MPA and LNG IUD
- Surgery: early local unroofing.
I&D only for pain relief from tense abscess. Follow with 3 gm load Amoxicillin/Clavulanate orally x 1, then 1 gm 3 x a day x 5-7 days. Follow with doxycycline 100-200 mg/day orally.
Treatment: Hurley Stage III is used in preparation for surgery, palliative
- Anti-inflammatory/antibiotics
Clindamycin 30 mg orally 2 x a day+ rifampin 300 mg orally 2 x a day
Prednisone 40-60 mg/day orally x 3-4 days, then taper over 7-10 d
Triamcinolone IM 1 mg/kg up to 60-80 mg intralesionally x 1
Cyclosporine 4-5 mg/kg/day orally for 5 to 30 wks - Ertapenem 1gm IV daily 6 weeks alone or followed by Moxifloxacin 400mg/d + clindamycin 300 mg bid + rifampicin 600mg/d X 6 weeks then moxifloxacin + rifampicin X 4 weeks
- Tumor Necrosis Factor Alpha Inhibitors
Infliximab (Remicade): IV infusion 5 mg/kg (available if patient has Crohn disease plus HS)
Adalimumab (Humira):Auto-injection 40 mg every other week
Etanercept (Enbrel): self-SQ injections 25-50 mg 1-2 X/wk - Surgery: Surgery is extensive, with special wound care and avoidance of primary closure. Deroofing the sinus tracts, removal of all granulation tissue and slow healing by secondary intention is effective. An effective technique is outlined by Danby in his article.21
PEARLS
HS is caused by follicular plugging, leading to follicular rupture and resulting in an inflammatory cascade which can result in painful inflamed nodules, draining sinus tracts, comedones, and scarring.
The diagnosis is based on typical lesions in typical intertriginous locations with recurrence and chronicity.
The diagnosis is often missed in its early stages, and is frequently misdiagnosed as recurrent furuncles or boils.
HS is not contagious, not caused by poor hygiene.
Weight loss and smoking cessation counseling should be recommended to all patients with HS as appropriate.
Antibiotics are used to decrease inflammation and to treat secondary infection. Systemic tetracyclines, alone or in combination with other agents, are frequently used as first line treatments.
Diet may be more valuable than surgery.
Hormone control is more valuable than antibiotics.
Surgery undertaken early in the disease need not be mutilating, expensive, or painful and is the only known curative procedure.
Tumor necrosis factor-alpha inhibitors are effective and safe for the treatment of HS. Adalimumab is the only therapy for HS approved by the U.S. Food and Drug Administration.
Systemic retinoids might decrease symptoms in younger patients with HS and acne and lower body weight.
References
- Wiltz O, Schoetz DJ Jr, Murray JJ, et al. Perianal hidradenitis suppurativa. The Lahey Clinic experience. Dis Colon Rectum.1990; 33:731.
- Ingram,JR. Hidradenitis suppurativa: pathogenesis, clinical features, and diagnosis. 2021 UpToDate, Inc. www.uptodate.com
- Wu,Y, et al. Nicotine enhances Staphylococcus epidermidis biofilm formation by altering the bacterial autolysis, extracellular DNA releasing and polysaccharide intercellular adhesin production. Front. Microbiol. 9:2575 (2018)
- Mortaz E, et al. Cigarette smoke induces CXCL8 production by human neutrophils via activation of TLR9 receptor. Eur Resp J 2010;36:1143
- Woodruff, CM, et al. Hidradenitis Suppuritiva: a Guide for the Practicing Physician. Mayo Clin Proc. December 2015;90(12):1679-1693)
- Margesson LJ and Danby FW. Hidradenitis suppurativa: Best Practice and Research. Clinical Obstetrics and Gynaecology 2014;28:1013-1027.
- Ingram JR. Hidradenitis suppurativa: Pathogenesis, clinical features, and diagnosis. 2021 UpToDate, Inc. www.uptodate.com
- Nguyen TV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities, and quality of life. JEADV 2021; 35:50-61
- Margesson LJ. Danby FW. Pathogenesis, clinical features, and diagnosis of hidradenitis suppurativa. In: Ofori, A, ed, UpToDate, Waltham, Massachusetts, 2011.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenighk RK, Roenigk HH, eds. Dermatologic surgery. Marcel Dekker, New York, 1989, 729-39.
- Layton A. Pathology of hidradenitis suppurativa. In: Jemec, GBE, Revuz J, Leyden JL, eds. Hidradenitis suppurativa. Springer, Berlin-Heidelberg, 2006, 27-30.
- Gemec GB, Gniadecka M. Sebum excretion in HS. Dermatol. 1997; 194:325.
- Sabat R, et al. Hidradenitis suppurativa. Nature Reviews/Disease Primers 2020;6:18
- Nguyen TV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities, and quality of life. JEADV 2021;35:50-61
- Nguyen TV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities, and quality of life. JEADV 2021;35:50-61
- Ingram JR. Hidradenitis suppurativa: Pathogenesis, clinical features, and diagnosis. 2021 UpToDate Inc.
- Sabat R, et al. Hidradenitis suppurativa. Nature Reviews/Disease Primers 2020;6:18
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010; 28:779.
- Lapins J, Emtestam L. Surgery. In: Jemec, GBE, Revuz J, Leyden JL, eds. Hidradenitis suppurativa. Springer, Berlin-Heidelberg, 2006, 163.
- Lapins J, Emtestam L. Surgery. In: Jemec, GBE, Revuz J, Leyden JL, eds. Hidradenitis suppurativa. Springer, Berlin-Heidelberg, 2006, 163
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010 Sep;63(3):481.e1-3