Introduction
Extramammary Paget disease (EMPD)1 is a rare intraepithelial adenocarcinoma of apocrine gland cells, found in the anogenital, umbilical, or axillary areas. The vulva is the most frequently involved site, with 65% of EMPD located in this region.2 The early course of the disease is indolent and, in some cases, EMPD may be present for 10-15 years before evidence of cancer or metastases appear. Invasive adenocarcinomas may be present within or beneath the surface lesion (4 to 17 percent in two series totaling 176 patients)3 and some patients with EMPD of the vulva may have synchronous neoplasms. Approximately 20-30 percent of these patients have a noncontiguous carcinoma (e.g. involving breast, rectum, bladder, urethra, cervix, or ovary.4 Early recognition of this condition is imperative.5
Epidemiology
EMPD is so rare that only a few hundred cases have been documented in the world’s literature. It comprises less than 1% of vulvar malignancies.6 EMPD is usually found in older, postmenopausal, Caucasian women, but there is a male predominance in Japan.7
Etiology
The exact cause is still unknown. The origin of the cancerous cells is variously thought to be from apocrine glands, anal or other mucosa, or epidermal cells with apocrine differentiation. The etiology likely differs between the primary and secondary forms. The primary form appears to arise from the epidermis, the cells being of sweat gland origin or from Toker cells.8 Toker cells are epidermal cells that share characteristics with Paget cells; both stain for mucin and are positive for CD 7.9 Toker cells also share the same distribution as apocrine glands: the breasts, milk line, and anogenital skin.10 Secondary EMPD arises from cells that migrate from the underlying adenocarcinoma.11 12
Symptoms and clinical features
Located on the vulva, (mons pubis, labia majora, labia minora, and perianal area) as well as inner thigh, EMPD presents with pruritus in 70 percent of women. Pruritus fails to clear with topical steroids. Pain or burning are less common complaints and some patients are asymptomatic.13 The lesion is typically a slowly expanding plaque that may have been present for some time, potentially misdiagnosed as eczema that is unresponsive to steroid application.
The clinical picture is varied. Usually there is an eczematous, erythematous papule or plaque ranging in size from one to 10 cm with a variable degree of scaling and a sharply demarcated border.
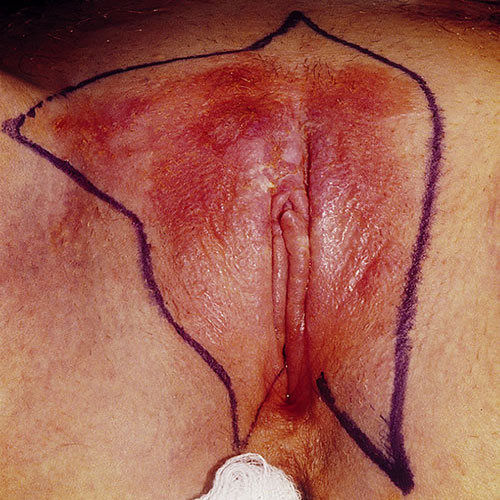
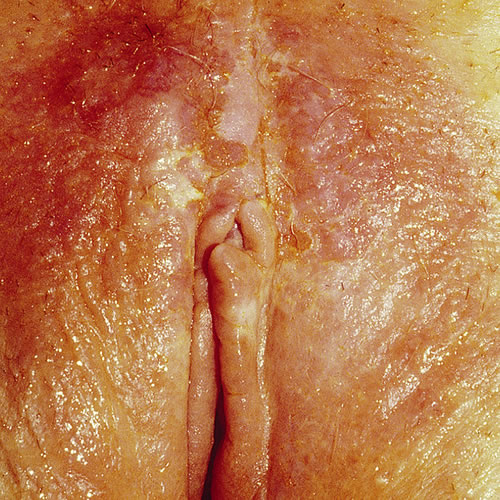
The surface may show erosion or mild crusting. If the involvement is near the mucosa and the area is moist, the moist dysplastic keratin of the lesion is white. At times, the lesions may form gyrate multifocal plaques. It is sometimes described as a “strawberries and cream” appearance when erosions and white scale are present. Dermoscopy may be helpful in diagnosis.14
Pathology/Laboratory Findings
Biopsy shows large clear cells with nuclear atypia and chronic inflammatory atypia, known as Paget cells.15
Diagnosis
Diagnosis is made on clinical suspicion supported by histopathology, including special stains.
Differential diagnosis
Differential diagnoses include malignant melanoma, HSIL or dVIN, psoriasis, erosive lichen planus, lichen simplex chronicus, Darier disease, Hailey-Hailey disease, tinea cruris, irritant or allergic contact dermatitis and candidiasis.
Treatment and management
Efforts should be made to detect any underlying sweat gland carcinoma or adenocarcinoma. Ruling out breast, genitourinary, perianal, and anal carcinoma is important. Investigations should include breast examination with mammography, barium studies, endoscopy of the entire gastrointestinal tract, IVP, bladder cytology, and a thorough vaginal examination with colposcopy, cervical cytology, and endometrial biopsy.
Wide local surgical excision with a 1.0 cm margin of normal skin is the standard treatment option for EMPD.16 17 Since recurrence rates between 16-50% are seen after regular surgery,18 Mohs micrographic surgery has become the preferred treatment modality for EMPD.19 But, recurrences rates as high as 27% following Mohs surgery have been reported20 and Mohs surgery may not be practical in the case of a very large lesion. Recently, a modified Mohs approach has been used effectively.21
Other treatment options that have been employed are CO2 laser ablation, electrodessication and curettage, photodynamic therapy, and radiotherapy, as well as topical use of bleomycin and 5-fluorouracil. Because there were low recurrence rates in radiotherapy treatment for EMPD primary lesions in more than one 2014 study, one author recommends it as a first line treatment when surgery is not recommended. 22
Immunotherapy with topical imiquimod 5% cream has been successfully used to treat isolated cases of EMPD. Imiquimod is a topically applied imidazoquinoline immunomodulator that binds to the toll-like receptor 7 and induces local production of proinflammatory chemokines and cytokines. Its use has been approved in the treatment of human papilloma virus induced warts. Treatment of primary or recurrent EMPD with imiquimod has been reported in a total of 17 patients, with a complete response reported in 15.23 The total treatment duration varied from 6 to 24 weeks with a regimen usually starting as a daily application and then tapering of the treatment to one application every other day. The longest follow-up period reported in the literature is 26 months (1 case).
In a study published in 2021, Preti et al. analyzed 122 vulvar EMPD patients and reported that the cancer-specific survival at 120 months was 100% for intraepithelial and microinvasive (≤ 1 mm) vulvar EMPD but 31% for invasive (>1 mm) vulvar EMPD.24 Similarly, van der Linden et al. analyzed 113 patients with vulvar EMPD and demonstrated that the 5-year disease-specific survival rate was greater than 98% in noninvasive and microinvasive EMPD, but was only 50% and significantly worse in invasive EMPD.25
Management requires the expertise of a gynecologic oncologist. Follow up is long term.
References
- Fisher BK, Margesson, LJ. Genital Skin Disorders: Diagnosis and Treatment. Mosby, Inc., 1998.188-189.
- McDaniel B, Brown F, Crane JS. Extramammary Paget Disease. [Updated 2021 Aug 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493224/
- Cooper S and Wojnarowska F. Anogenital (Non-venereal) Disease. Dermatology, edited by Jean Bolognia, Elsevier Limited, 2018
- Feuer GA, Shevchuk M, Calanog A. Vulvar Paget’s Disease: the need to exclude an invasive lesion. Am J Dermatopathol. 2005;27:185-188.
- McDaniel B, Brown F, Crane JS. Extramammary Paget Disease. [Updated 2021 Aug 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493224/
- Parker LP, Parker JR, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. SOGynecol Oncol. 2000;77(1):183.
- Cooper S and Wojnarowska F. “Anogenital (Non-venereal) Disease.” Dermatology, edited by Jean Bolognia, Elsevier Limited, 2018.
- William JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Liegl B, Leibl S, gogg-Kamerer M, et al. Mammary and extramammary Paget’s disease: an immunohistochemical study of 83 cases. Histopathology. 2007;50:439-447.
- Di Tommaso L, Franchi G, Destro A, et al. Toker cells of the breast: morphological and immunohistochemical characterization of 40 cases. Hum Pathol. 2008; 39:1295-1300.
- Edwards L and Lynch P. Genital Dermatology Atlas, second edition. Wolters Kluwer Health/Lippincott, Williams and Wilkins, Philadelphia. 2011. 81.
- McDaniel B, Brown F, Crane JS. Extramammary Paget Disease. [Updated 2021 Aug 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493224/
- St Claire K., Hoover A., Ashack K., Khachemoune A. Extramammary Paget disease. Dermatol. Online J. 2019;25:4. doi: 10.5070/D3254043591.
- Mun J.H., Park S.M., Kim G.W., Song M., Kim H.S., Ko H.C., Kim B.S., Kim M.B. Clinical and dermoscopic characteristics of extramammary Paget disease: A study of 35 cases. Br. J. Dermatol. 2016;174:1104–1107. doi: 10.1111/bjd.14300.
- Edwards L and Lynch P. Genital Dermatology Atlas, second edition. Wolters Kluwer Health/Lippincott, Williams and Wilkins, Philadelphia. 2011. 80.
- Murata Y., Kumano K. Extramammary Paget’s disease of the genitalia with clinically clear margins can be adequately resected with 1 cm margin. Eur. J. Dermatol. 2005;15:168–170.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742–749.
- Zampogna JC, Flowers FP, Roth WI, Hassenein AM. Treatment of primary limited cutaneous extramammary Paget’s disease with topical imiquimod monotherapy: two case reports. J Am Acad Dermatol. 2002;47(4 Suppl):S229–235
- Gunn RA, Gallager HS. Vulvar Paget’s disease: a topographic study. Cancer. 1980;46:590–594.
- Hendi A, Brodland DG, Zitelli JA. Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51:767–773.
- Chang M.S., Mulvaney P.M., Danesh M.J., Feltmate C.M., Schmults C.D. Modified peripheral and central Mohs micrographic surgery for improved margin control in extramammary Paget disease. JAAD Case Rep. 2021;7:71–73. doi: 10.1016/j.jdcr.2020.11.002.
- Ishizuki S, Nakamura Y. Extramammary Paget’s Disease: Diagnosis, Pathogenesis, and Treatment with Focus on Recent Developments. Curr Oncol. 2021;28(4):2969-2986. Published 2021 Aug 5. doi:10.3390/curroncol28040260
- Feldmeyer L, Kerl K, Kamarashev J, de Viragh P, French LE. Treatment of vulvar Paget disease with topical imiquimod: a case report and review of the literature. J Dermatol Case Rep. 2011 Sep 21;5(3):42-6.
- Preti M., Micheletti L., Borella F., Cosma S., Marrazzu A, et al. Vulvar Paget’s disease and stromal invasion: Clinico-pathological features and survival outcomes. Surg. Oncol. 2021;38:101581. doi: 10.1016/j.suronc.2021.101581
- van der Linden M., Oonk M.H.M., et al. Vulvar Paget disease: A national retrospective cohort study. J. Am. Acad. Dermatol. 2019;81:956–962. doi: 10.1016/j.jaad.2018.11.016.